We serve Chemical Name:N-3 Fondaparinux CAS:114903-05-8 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
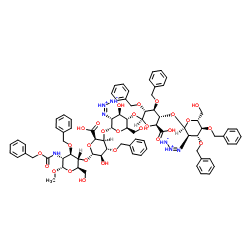
Chemical Name:N-3 Fondaparinux
CAS.NO:114903-05-8
Synonyms:Methyl 3,4-di-O-benzyl-2-deoxy-2-[(1Z)-1-triazen-2-ium-3-id-1-yl]-α-D-glucopyranosyl-(1->4)-2,3-di-O-benzyl-β-D-glucopyranuronosyl-(1->4)-2-deoxy-2-[(1Z)-1-triazen-2-ium-3-id-1-yl]-α-D-gluc opyranosyl-(1->4)-3-O-benzyl-α-L-idopyranuronosyl-(1->4)-3-O-benzyl-2-{[(benzyloxy)carbonyl]amino}-2-deoxy-α-D-glucopyranoside;Methyl O-2-azido-2-deoxy-3,4-bis-O-(phenylmethyl)-alpha-D-glucopyranosyl-(1→4)-O-2,3-bis-O-(phenylmethyl)-beta-D-glucopyranuronosyl-(1→4)-O-2-azido-2-deoxy-alpha-D-glucopyranosyl-(1→4)-O-3-O-(phenylmethyl)-alpha-L-idopyranuronosyl-(1→4)-2-deoxy-2-[[(phenylmethoxy)carbonyl]amino]-3-O-(phenylmethyl)-alpha-D-glucopyranoside
Molecular Formula:C81H91N7O27
Molecular Weight:1598.652
HS Code:
Physical and Chemical Properties:
Melting point:N/A
Boiling point:N/A
Density:N/A
Index of Refraction:
PSA:
Exact Mass:1597.627563
LogP:
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like Methyl 3,4-di-O-benzyl-2-deoxy-2-[(1Z)-1-triazen-2-ium-3-id-1-yl]-α-D-glucopyranosyl-(1-> chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,Methyl O-2-azido-2-deoxy-3,4-bis-O-(phenylmethyl)-alpha-D-glucopyranosyl-(1→4)-O-2,3-bis-O-(phenylmethyl)-beta-D-glucopyranuronosyl-(1→4)-O-2-azido-2-deoxy-alpha-D-glucopyranosyl-(1→4)-O-3-O-(phenylmethyl)-alpha-L-idopyranuronosyl-(1→4)-2-deoxy-2-[[(phenylmethoxy)carbonyl]amino]-3-O-(phenylmethyl)-alpha-D-glucopyranoside physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,4)-2-deoxy-2-[(1Z)-1-triazen-2-ium-3-id-1-yl]-α-D-gluc opyranosyl-(1-> Use and application,4)-3-O-benzyl-2-{[(benzyloxy)carbonyl]amino}-2-deoxy-α-D-glucopyranoside technical grade,usp/ep/jp grade.
Related News: The Ballina facility was first established in 1974 and was acquired by Charles River in 2002. Over the years it has added to its capabilities offering a comprehensive package of GMP services in support of recombinant biologics, vaccines, cell and gene therapies, biosimilars, and medical devices. Charles River now employs 230 people at two facilities in Ireland: the site in Ballina Co. Mayo which focuses on biologics testing, and a site in Dublin, established in 2017, which serves as the EMEA and APAC headquarters for the Company’s Microbial Solutions division. quinoxaline-2,3-dicarboxylic acid manufacturers “His strong track record of success and deep experience of fast-moving consumer goods and consumer health, proven at P&G, Novartis and GSK, means he is the right choice” to lead the consumer health unit as an independent company, GSK Chairman Jonanthan Symonds said of McNamara in the statement. 3-amino-5-(4-(bis(4-fluorophenyl)methyl)piperazin-1-yl)-4′-chloro-[1,1′-biphenyl]-2,4-dicarbonitrile suppliers Earlier this year, the company revealed it had broken ground on a 38,000-square-foot commercial cell therapy plant in Lexington, Massachusetts, which is about 10 miles from downtown Boston. Takeda says the new $84 million plant will be used to make cell therapies for cancers and other diseases. cis-[(pentafluorophenyl)(triphenylphosphine)Pt(μ-H)(μ-pentafluorophenyl)Pt(pentafluorophenyl)(triphenylphosphine)] vendor & factory The government has approved a total of 33 applications with a committed investment of Rs 5,082.65 crore under the production linked incentive scheme for active pharmaceutical ingredients, an official release said on Thursday.,The Ballina facility was first established in 1974 and was acquired by Charles River in 2002. Over the years it has added to its capabilities offering a comprehensive package of GMP services in support of recombinant biologics, vaccines, cell and gene therapies, biosimilars, and medical devices. Charles River now employs 230 people at two facilities in Ireland: the site in Ballina Co. Mayo which focuses on biologics testing, and a site in Dublin, established in 2017, which serves as the EMEA and APAC headquarters for the Company’s Microbial Solutions division.

