We serve Chemical Name:4-(4-Iodophenyl)pyridine CAS:83420-59-1 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
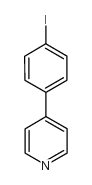
Chemical Name:4-(4-Iodophenyl)pyridine
CAS.NO:83420-59-1
Synonyms:Pyridine,4-(4-iodophenyl);4-(4′-iodophenyl)pyridine
Molecular Formula:C11H8IN
Molecular Weight:281.09200
HS Code:2933399090
Physical and Chemical Properties:
Melting point:204ºC
Boiling point:N/A
Density:1.653
Index of Refraction:
PSA:12.89000
Exact Mass:280.97000
LogP:3.35320
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like Pyridine,4-(4-iodophenyl) chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,4-(4′-iodophenyl)pyridine physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,Pyridine,4-(4-iodophenyl) Use and application,Pyridine,4-(4-iodophenyl) technical grade,usp/ep/jp grade.
Related News: It has reserves in the direction of APIs and intermediates for lowering blood lipids, lowering blood sugar and anticoagulation. 4-(4-Iodophenyl)pyridine manufacturer It has reserves in the direction of APIs and intermediates for lowering blood lipids, lowering blood sugar and anticoagulation. 4-(4-Iodophenyl)pyridine supplier At present, there are more than 8,000 domestic API manufacturers, but they mainly produce bulk APIs with low technical content. 4-(4-Iodophenyl)pyridine vendor At present, Teva can produce more than 300 generic drugs, and the API department has approximately 650 authorized patents and patent applications worldwide. It is also the generic drug company with the most challenges in patenting ParagraphIV in the world. 4-(4-Iodophenyl)pyridine factory The GLOW trial doesn’t yet have the answer to that question, but Tendler pointed to encouraging early evidence from the regimen’s phase 2 trial, dubbed CAPTIVATE. Among eight patients who progressed after stopping the fixed-duration combo, six responded to subsequent Imbruvica monotherapy, while the other two didn’t have a response report, according to data presented at the recent American Society of Clinical Oncology annual meeting.

