We serve Chemical Name:Sodium 1-undecanesulfonate CAS:5838-34-6 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
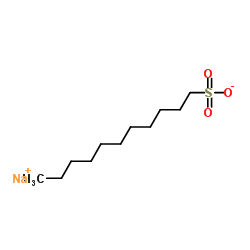
Chemical Name:Sodium 1-undecanesulfonate
CAS.NO:5838-34-6
Synonyms:Sodium undecane-1-sulfonate;sodium,undecane-1-sulfonate;1-Undecanesulfonic acid, sodium salt (1:1);MFCD00053627;Sodium 1-undecanesulfonate
Molecular Formula:C11H23NaO3S
Molecular Weight:258.353
HS Code:
Physical and Chemical Properties:
Melting point:>300°C
Boiling point:N/A
Density:1.547g/cm3
Index of Refraction:
PSA:65.58000
Exact Mass:258.126556
LogP:4.14320
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like Sodium undecane-1-sulfonate chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,Sodium 1-undecanesulfonate physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,MFCD00053627 Use and application,1-Undecanesulfonic acid, sodium salt (1:1) technical grade,usp/ep/jp grade.
Related News: We are pleased to work with Inceptua, given their strong record of administering such programs successfully.�� Sodium 1-undecanesulfonate manufacturer This means that the drug attributes of the drug substance will be lost in the future, and the monopoly power of some drug substances will also be lost. The preparation company will become the main person in charge of the drug. The drug preparation company will be responsible for the quality of the original excipients. It will be more cautious, some raw and auxiliary materials companies whose quality cannot be guaranteed will be gradually eliminated, and the industry concentration will be further improved. Sodium 1-undecanesulfonate supplier The agency said it was not yet ready to authorize Emergent BioSolutions Inc’s (EBS.N) plant for manufacturing the J&J vaccine. Sodium 1-undecanesulfonate vendor This means that the drug attributes of the drug substance will be lost in the future, and the monopoly power of some drug substances will also be lost. The preparation company will become the main person in charge of the drug. The drug preparation company will be responsible for the quality of the original excipients. It will be more cautious, some raw and auxiliary materials companies whose quality cannot be guaranteed will be gradually eliminated, and the industry concentration will be further improved. Sodium 1-undecanesulfonate factory Taiwan complained on Sunday that it was being punished because the World Health Organization considers it part of China, which has been subject to travel bans as the coronavirus spreads.

