We serve Chemical Name:1,8-Naphthyridine-2-carboxylic acid CAS:215523-34-5 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
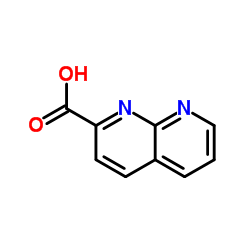
Chemical Name:1,8-Naphthyridine-2-carboxylic acid
CAS.NO:215523-34-5
Synonyms:1,8-Naphthyridine-2-carboxylic acid;1,8-naphthyridin-2-carboxylic acid
Molecular Formula:C9H6N2O2
Molecular Weight:174.156
HS Code:2933990090
Physical and Chemical Properties:
Melting point:N/A
Boiling point:366.8±27.0 °C at 760 mmHg
Density:1.4±0.1 g/cm3
Index of Refraction:1.697
PSA:63.08000
Exact Mass:174.042923
LogP:1.00
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like 1,8-Naphthyridine-2-carboxylic acid chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,1,8-naphthyridin-2-carboxylic acid physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,1,8-Naphthyridine-2-carboxylic acid Use and application,1,8-Naphthyridine-2-carboxylic acid technical grade,usp/ep/jp grade.
Related News: With the chemical industry being one of the most polluting, global efforts are heavily focused on developing a more sustainable and greener footprint. (2S)-2-amino-3-[3-[(2-arsonophenyl)hydrazinylidene]-4-oxocyclohexa-1,5-dien-1-yl]propanoic acid manufacturers This has led to them being evaluated as alternatives to traditional metal catalysts for driving various chemical transformations, where they have demonstrated huge success. Some of the first enzyme classes used as tools for organic synthesis were hydrolases (e.g. amidases, esterases, and lipases), alcohol dehydrogenases, and ketoreductases. 5-(4-chloro-phenyl)-10-methyl-2,3,5,10-tetrahydro-imidazo[2,1-b]quinazolin-5-ol suppliers AstraZeneca’s application for anifrolumab in SLE is under review by regulatory authorities in the US, EU and Japan, with decisions anticipated in the second half of 2021. Anifrolumab is not currently approved in any country. (2S)-2-[1-(3,4-dihydroxycyclopentyl)propylamino]-3-(1-methylindol-3-yl)propanoic acid vendor & factory.

