We serve Hydroxylamine sulfate CAS:10039-54-0 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
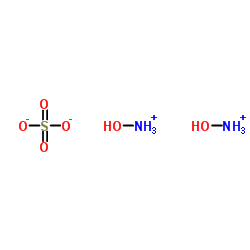
Contact us for information like Hydroxylamine sulfate chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,bis(hydroxylammonium) physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,Oxyammonium sulfate Use and application,Oxyammonium sulfate technical grade,usp/ep/jp grade.
Related News: China has asked the European Union for help in buying urgently needed medical supplies from its member countries, the China’s official Xinhua news agency said on Saturday.Diisopropyl Phosphonate manufacturer By drawing these distinctions between APIs and the drugs themselves, manufacturers are able to specialize and pharmacists able to align generic equivalents with brand names.1-[3-(2-aminoethyl)-1H-indol-5-yl]-N-methylmethanesulfonamide supplier These include: high-barrier generic drug substances and commonly used generic drug substances.L-alpha-Amino-n-butyric acid vendor “We will continue to closely monitor the situation and we look forward to reopening our stores as soon as possible.”Process: The importance of safety is obvious. The research and development of specialty drug substances (especially high-barrier generic drug substance drugs) usually need to avoid the original process patents, and some chemicals are developed because of the complex structure or the harsh synthetic conditions The synthetic route is more difficult, so the importance of process design capabilities of API companies is becoming more important.

