We serve 2,5-Dichloro-4,6-dimethylnicotinonitrile CAS:91591-63-8 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
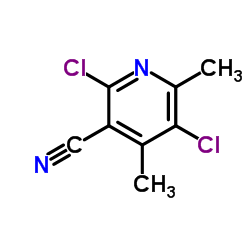
Contact us for information like 2,5-Dichloro-4,6-Dimethylnicotinonitrile chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,3-Pyridinecarbonitrile, 2,5-dichloro-4,6-dimethyl- physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,3-Pyridinecarbonitrile, 2,5-dichloro-4,6-dimethyl- Use and application,2,5-dichloro-4,6-dimethylpyridine-3-carbonitrile technical grade,usp/ep/jp grade.
Related News: The workers are demanding that Hong Kong close all border checkpoints to visitors from mainland China, saying they represent a threat to health care workers in the city.1-Chloro-4-fluorobutane manufacturer In 2016, the export volume of China’s specialty drug substances reached US $ 3.53 billion, accounting for 13.8% of the total drug substances.Ethyl (E)-hex-2-enoate supplier On Friday, the Trump administration announced it will deny entry to foreign nationals who have traveled in China in the last 14 days, starting Sunday.5-Cyanoindole vendor Beta Bionics, Inc. recently announced it has received Breakthrough Device designation from the US FDA for its investigational iLet Bionic Pancreas System.As proof-of-principle for the unique advantages arising from selecting a single engineered iPSC clone for the production of CAR T-cell therapy, the scientists assessed 747 clones after engineering a pool of cells using CRISPR. It was found that only about 2% of clones met the Company’s standards for overall quality including containing both bi-allelic disruption of the TCR, proper insertion of the CAR into the TRAC locus without random transgene integrations, and no evidence of off-target genomic modifications or translocations.

