We serve 2′-Deoxyuridine 5′-mono-phos-phate disodium salt CAS:42155-08-8 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
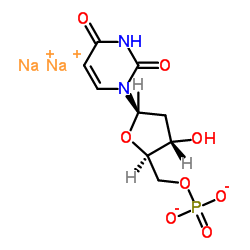
Contact us for information like 2′-Deoxyuridine 5′-mono-phos-phate disodium salt chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,Molecular Formula:C9H11N2Na2O8P physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,Molecular Formula:C9H11N2Na2O8P Use and application,2′-Deoxyuridine 5′-mono-phos-phate disodium salt technical grade,usp/ep/jp grade.
Related News: Madeleine Roche, Associate Pharmaceutical Analyst at GlobalData, comments: “Within the top 20 global companies that spent the most on R&D in 2018, the top spender – despite having the second smallest annual revenue of the group at $15.28 billion – is Celgene, whose R&D spend was equal to 37% of its annual revenue.7-hydroxy-1H-quinolin-2-one manufacturer Madeleine Roche, Associate Pharmaceutical Analyst at GlobalData, comments: “Within the top 20 global companies that spent the most on R&D in 2018, the top spender – despite having the second smallest annual revenue of the group at $15.28 billion – is Celgene, whose R&D spend was equal to 37% of its annual revenue.4-(4-Bromo-3-formylphenoxy)benzonitrile supplier Onconova is currently in the clinical development stage with oral and IV rigosertib, including clinical trials studying single agent IV rigosertib in second-line higher-risk MDS patients (pivotal Phase 3 INSPIRE trial) and oral rigosertib plus azacitidine in first-line and refractory higher-risk MDS patients (Phase 2).2-(2-methyl-1H-indol-3-yl)ethanamine vendor Onconova is currently in the clinical development stage with oral and IV rigosertib, including clinical trials studying single agent IV rigosertib in second-line higher-risk MDS patients (pivotal Phase 3 INSPIRE trial) and oral rigosertib plus azacitidine in first-line and refractory higher-risk MDS patients (Phase 2).Onconova is currently in the clinical development stage with oral and IV rigosertib, including clinical trials studying single agent IV rigosertib in second-line higher-risk MDS patients (pivotal Phase 3 INSPIRE trial) and oral rigosertib plus azacitidine in first-line and refractory higher-risk MDS patients (Phase 2).

