We serve Chemical Name:6-Chloro-1H-indol-4-amine CAS:431046-15-0 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
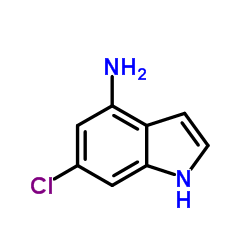
Chemical Name:6-Chloro-1H-indol-4-amine
CAS.NO:431046-15-0
Synonyms:1H-Indol-4-amine, 6-chloro-;6-Chloro-1H-indol-4-amine
Molecular Formula:C8H7ClN2
Molecular Weight:166.608
HS Code:2933990090
Physical and Chemical Properties:
Melting point:N/A
Boiling point:393.8±22.0 °C at 760 mmHg
Density:1.4±0.1 g/cm3
Index of Refraction:1.758
PSA:41.81000
Exact Mass:166.029770
LogP:1.74
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like 1H-Indol-4-amine, 6-chloro- chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,6-Chloro-1H-indol-4-amine physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,6-Chloro-1H-indol-4-amine Use and application,6-Chloro-1H-indol-4-amine technical grade,usp/ep/jp grade.
Related News: The European Medicines Agency did not say how many shots were affected, but Reuters has reported it involves millions of doses, making it harder for J&J to meet a target of delivering 55 million to Europe by end of June. 6-Chloro-1H-indol-4-amine manufacturer The FDA approval allows Biogen to sell its product over several years – with forecasts for potential annual sales reaching as high $10 billion to $50 billion – until the company completes a required follow-up study. 6-Chloro-1H-indol-4-amine supplier The FDA approval allows Biogen to sell its product over several years – with forecasts for potential annual sales reaching as high $10 billion to $50 billion – until the company completes a required follow-up study. 6-Chloro-1H-indol-4-amine vendor Rigosertib is a small molecule that inhibits cellular signaling in cancer cells by acting as a RAS mimetic. Current clinical development of rigosertib is centered upon the therapeutic management of MDS, a heterogeneous group of bone marrow disorders characterized by ineffective hematopoiesis that often develop into acute myeloid leukaemia (AML). 6-Chloro-1H-indol-4-amine factory The results of analysis perfectly align with the tau hypothesis — simply put, if the patient is tau biomarker positive, then tau pathology is responsible for his/her cognitive decline, and halting tau pathology should slow or halt progression,” Novak said. “If the patient is negative for markers of tau pathology, then this patient’s impairment is mainly due to other pathologies, and treating tau pathology in this patient won’t be meaningful.

