We serve Chemical Name:4-(1H-Pyrazol-4-yl)pyrimidine CAS:28648-87-5 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
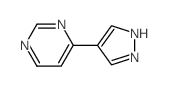
Chemical Name:4-(1H-Pyrazol-4-yl)pyrimidine
CAS.NO:28648-87-5
Synonyms:4-Pyrazolylpyrimidine;HMS523N07;4-pyrazol-4-ylpyrimidine
Molecular Formula:C7H6N4
Molecular Weight:146.14900
HS Code:2933990090
Physical and Chemical Properties:
Melting point:205-208℃
Boiling point:N/A
Density:N/A
Index of Refraction:
PSA:54.46000
Exact Mass:146.05900
LogP:0.86670
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like 4-Pyrazolylpyrimidine chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,4-pyrazol-4-ylpyrimidine physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,HMS523N07 Use and application,4-Pyrazolylpyrimidine technical grade,usp/ep/jp grade.
Related News: The New York Times said that the batches being discarded amount to around 60 million doses, citing people familiar with the matter. 4-(1H-Pyrazol-4-yl)pyrimidine manufacturer The KCDC said a 28-year-old man had tested positive. He was among the 368 repatriated South Koreans who arrived from Wuhan on a charter flight on January 31. The rest of the evacuees have tested negative for the coronavirus. 4-(1H-Pyrazol-4-yl)pyrimidine supplier The government will also send a charter flight with an Air New Zealand crew to repatriate up to 300 citizens in Wuhan. 4-(1H-Pyrazol-4-yl)pyrimidine vendor The KCDC said a 28-year-old man had tested positive. He was among the 368 repatriated South Koreans who arrived from Wuhan on a charter flight on January 31. The rest of the evacuees have tested negative for the coronavirus. 4-(1H-Pyrazol-4-yl)pyrimidine factory The memo denies the company made false statements to the FDA. In their complaint, employees said they were broadly concerned that quality control documents the FDA requires companies to maintain had been rewritten or fabricated. The employees did not specify whether these materials had been shown to the FDA.

