We serve Chemical Name:pentanedioic acid,propane-1,3-diol CAS:52256-48-1 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
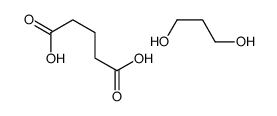
Chemical Name:pentanedioic acid,propane-1,3-diol
CAS.NO:52256-48-1
Synonyms:MFCD00274740;Poly(1,3-propylene glutarate)
Molecular Formula:C8H16O6
Molecular Weight:208.20900
HS Code:
Physical and Chemical Properties:
Melting point:N/A
Boiling point:N/A
Density:N/A
Index of Refraction:
PSA:115.06000
Exact Mass:208.09500
LogP:
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:NONH for all modes of transpor
Packing Group:
Contact us for information like MFCD00274740 chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,Poly(1,3-propylene glutarate) physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,MFCD00274740 Use and application,Poly(1,3-propylene glutarate) technical grade,usp/ep/jp grade.
Related News: Events, Hatzalah of South Florida, and Ygrene and in partnership with the Shul of Bal Harbour we are able to continue this mission by providing first and last month rent as well as the security deposit for these families If this fund grows bigger, we will be able to continue to contribute funds directly for the rents.” Michael Capponi
“During this challenging time for the survivors, the uncertainty of not knowing where they will live exacerbates an already extremely painful and tragic situation. We are committed to the relocation efforts of all of the survivors through our partnership with Global Empowerment Mission.” Rabbi Zalman Lipskar, The Shul of Bal Harbour.
“We are devastated by the loss suffered by the victims, families of victims and the community at the hands of the Surfside tragedy. It is an honor to contribute to GEM’s Champlain Survivor Relocation Fund to provide safe and comfortable homes for survivors to help them get back on their feet, ” said Ygrene CEO, Jim Reinhart.
Baclofen is a muscle relaxant drug indicated for the treatment of muscle pain, spasms, and stiffness in people with multiple sclerosis, spinal cord injury, or disease.
This is Beximco Pharma’s ninth Abbreviated New Drug Application fully developed in-house and successfully approved for the US market since the Company’s oral solid dosage facility was approved by the US FDA in June 2015.
According to IQVIA audited market data, there are currently nine active players for Baclofen in the US market, which generated sales of more than $110 million in 2020.
Mr. Nazmul Hassan MP, Managing Director of Beximco Pharmaceuticals, said: We are pleased to have received FDA approval of Baclofen, a commonly prescribed medicine in the US, as we continue to leverage our core strengths in R&D and manufacturing to develop and deliver important generic products to patients. carboxymethyl-1 N-acetyl-2 [hydroxy-3 (tri-O-benzoyl-2′,3′,5′ β-D-ribofuranosyl)-4 phenyl]-3 alanine manufacturers According to the Food and Drug Administration and the Orphan Drug Designation program, orphan status applies to drugs and biologics defined as “those intended for the safe and effective treatment, diagnosis or prevention of rare diseases/disorders that affect fewer than 200,000 people in the US, or that affect more than 200,000 people, 2-fluoro-4-(2-((1r,4s)-4-(hexyloxy)cyclohexyl)ethyl)-1-isothiocyanatobenzene suppliers Inspired by the spirit of Start with Integrity, Succeed through Action,” Innovent’s mission is to develop, manufacture and commercialize high-quality biopharmaceutical products that are affordable to ordinary people.
Established in 2011, Innovent is committed to developing, manufacturing and commercializing high-quality innovative medicines for the treatment of cancer, autoimmune, metabolic and other major diseases. On October 31, 2018, Innovent was listed on the Main Board of the Stock Exchange of Hong Kong Limited with the stock code: 01801.HK.
Since its inception, Innovent has developed a fully integrated multi-functional platform which includes R&D, CMC (Chemistry, Manufacturing, and Controls), clinical development and commercialization capabilities.
Leveraging the platform, the company has built a robust pipeline of 25 valuable assets in the fields of cancer, metabolic, autoimmune disease and other major therapeutic areas, with 5 products – TYVYT® (sintilimab injection), BYVASDA® (bevacizumab biosimilar injection), SULINNO® (adalimumab biosimilar injection), HALPRYZA® (rituximab biosimilar injection) and Pemazyre® (pemigatinib oral inhibitor) – officially approved for marketing, 1 asset’s NDA under NMPA review, sintilimab’s Biologics License Application (BLA) acceptance in the U.S., 5 assets in Phase 3 or pivotal clinical trials, and an additional 14 molecules in clinical studies.
Innovent has built an international team with advanced talent in high-end biological drug development and commercialization, including many global experts. The company has also entered into strategic collaborations with Eli Lilly and Company, Adimab, Incyte, MD Anderson Cancer Center, Hanmi and other international partners.
Innovent strives to work with many collaborators to help advance China’s biopharmaceutical industry, improve drug availability and enhance the quality of the patients’ lives. For more information, please visit 4-(3-(5-(tert-butyl)thiophen-2-yl)-2-methylpropyl)-2,6-dimethylmorpholine hydrochloride vendor & factory.
“During this challenging time for the survivors, the uncertainty of not knowing where they will live exacerbates an already extremely painful and tragic situation. We are committed to the relocation efforts of all of the survivors through our partnership with Global Empowerment Mission.” Rabbi Zalman Lipskar, The Shul of Bal Harbour.
“We are devastated by the loss suffered by the victims, families of victims and the community at the hands of the Surfside tragedy. It is an honor to contribute to GEM’s Champlain Survivor Relocation Fund to provide safe and comfortable homes for survivors to help them get back on their feet, ” said Ygrene CEO, Jim Reinhart.
Baclofen is a muscle relaxant drug indicated for the treatment of muscle pain, spasms, and stiffness in people with multiple sclerosis, spinal cord injury, or disease.
This is Beximco Pharma’s ninth Abbreviated New Drug Application fully developed in-house and successfully approved for the US market since the Company’s oral solid dosage facility was approved by the US FDA in June 2015.
According to IQVIA audited market data, there are currently nine active players for Baclofen in the US market, which generated sales of more than $110 million in 2020.
Mr. Nazmul Hassan MP, Managing Director of Beximco Pharmaceuticals, said: We are pleased to have received FDA approval of Baclofen, a commonly prescribed medicine in the US, as we continue to leverage our core strengths in R&D and manufacturing to develop and deliver important generic products to patients. carboxymethyl-1 N-acetyl-2 [hydroxy-3 (tri-O-benzoyl-2′,3′,5′ β-D-ribofuranosyl)-4 phenyl]-3 alanine manufacturers According to the Food and Drug Administration and the Orphan Drug Designation program, orphan status applies to drugs and biologics defined as “those intended for the safe and effective treatment, diagnosis or prevention of rare diseases/disorders that affect fewer than 200,000 people in the US, or that affect more than 200,000 people, 2-fluoro-4-(2-((1r,4s)-4-(hexyloxy)cyclohexyl)ethyl)-1-isothiocyanatobenzene suppliers Inspired by the spirit of Start with Integrity, Succeed through Action,” Innovent’s mission is to develop, manufacture and commercialize high-quality biopharmaceutical products that are affordable to ordinary people.
Established in 2011, Innovent is committed to developing, manufacturing and commercializing high-quality innovative medicines for the treatment of cancer, autoimmune, metabolic and other major diseases. On October 31, 2018, Innovent was listed on the Main Board of the Stock Exchange of Hong Kong Limited with the stock code: 01801.HK.
Since its inception, Innovent has developed a fully integrated multi-functional platform which includes R&D, CMC (Chemistry, Manufacturing, and Controls), clinical development and commercialization capabilities.
Leveraging the platform, the company has built a robust pipeline of 25 valuable assets in the fields of cancer, metabolic, autoimmune disease and other major therapeutic areas, with 5 products – TYVYT® (sintilimab injection), BYVASDA® (bevacizumab biosimilar injection), SULINNO® (adalimumab biosimilar injection), HALPRYZA® (rituximab biosimilar injection) and Pemazyre® (pemigatinib oral inhibitor) – officially approved for marketing, 1 asset’s NDA under NMPA review, sintilimab’s Biologics License Application (BLA) acceptance in the U.S., 5 assets in Phase 3 or pivotal clinical trials, and an additional 14 molecules in clinical studies.
Innovent has built an international team with advanced talent in high-end biological drug development and commercialization, including many global experts. The company has also entered into strategic collaborations with Eli Lilly and Company, Adimab, Incyte, MD Anderson Cancer Center, Hanmi and other international partners.
Innovent strives to work with many collaborators to help advance China’s biopharmaceutical industry, improve drug availability and enhance the quality of the patients’ lives. For more information, please visit 4-(3-(5-(tert-butyl)thiophen-2-yl)-2-methylpropyl)-2,6-dimethylmorpholine hydrochloride vendor & factory.

