We serve Chemical Name:2-Chloro-3-(1-pyrrolidinyl)pyrazine CAS:1209459-63-1 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
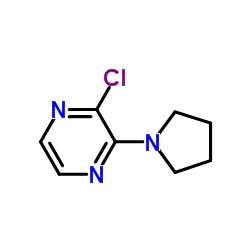
Chemical Name:2-Chloro-3-(1-pyrrolidinyl)pyrazine
CAS.NO:1209459-63-1
Synonyms:Pyrazine, 2-chloro-3-(1-pyrrolidinyl)-;PYRAZINE,2-CHLORO-3-(1-PYRROLIDINYL);2-Chloro-3-(1-pyrrolidinyl)pyrazine;2-chloro-3-(pyrrolidin-1-yl)pyrazine
Molecular Formula:C8H10ClN3
Molecular Weight:183.638
HS Code:2933990090
Physical and Chemical Properties:
Melting point:N/A
Boiling point:292.8±40.0 °C at 760 mmHg
Density:1.3±0.1 g/cm3
Index of Refraction:1.582
PSA:29.02000
Exact Mass:183.056320
LogP:2.34
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like Pyrazine, 2-chloro-3-(1-pyrrolidinyl)- chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,2-chloro-3-(pyrrolidin-1-yl)pyrazine physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,Pyrazine, 2-chloro-3-(1-pyrrolidinyl)- Use and application,2-Chloro-3-(1-pyrrolidinyl)pyrazine technical grade,usp/ep/jp grade.
Related News: “The Agency also noted that no effect was seen in the two studies that included patients from EU populations, including the most recent study which involved patients who were receiving the maximum and optimal treatment for their Parkinson’s disease,” EMA said. niobium boride manufacturers Cholesterol drug Crestor, blood thinner Brilinta and acid reflux med Nexium have all suffered as a result. Brilinta, for example, saw emerging markets sales drop 40% at constant currencies to $180 million in the first six months of 2021. [4-(prop-2-enylcarbamoyl)phenyl]boronic acid suppliers Conducted by Innovent in China, the CIBI321A101 trial is a Phase 1a open-label, multi-center study of the safety, tolerability and primary efficacy of IBI321 in patients with advanced solid tumors. Phase 1a of the study will evaluate dosing of IBI321 in a variety of solid tumors (ClinicalTrials.gov, NCT04911894). 2-Benzothiazolamine,N-ethyl-(9CI) vendor & factory.

