We serve 3-Bromo-5-fluoropyridine CAS:407-20-5 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
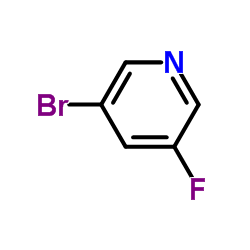
Contact us for information like 3-Bromo-5-fluoropyridine chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,3-bromo-5-fluoropyridine physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,3-BroMo-5-fluoropyridine Use and application,3-bromo-5-fluoropyridine technical grade,usp/ep/jp grade.
Related News: South Korea and Japan are barring noncitizens who traveled recently to Hubei, the province at the center of the outbreak.Ethyl 2,3-dibromopropionate manufacturer By recapitulating the tumor microenvironment and using a live-cell, function-first approach, Resonant’s platform generates therapeutic candidates that would not be discovered by other methods with unprecedented speed.2-Methoxy-5-methylpyridine supplier The rest of South Korea will also consider stopping issuing tourist visas to Chinese nationals, said Park Neung-hoo, the government’s minister of health and welfare. All travelers from China will be separated from the other arrivals for heightened screening at airports.N-(4-ethoxyphenyl)-3-hydroxynaphthalene-2-carboxamide vendor After several years of R & D and improvement, the production process of commonly used generic drug bulk drugs is relatively mature, and the products of similar companies have high similarities. Therefore, the competitive advantage of bulk drug companies is mainly reflected in cost control. Companies with cost advantages can usually pass Competition to expand production capacity and further gain scale advantages, while having stable, high-quality upstream supply.The acquired pharmaceutical intermediates business will be renamed Corden Pharma Switzerland LLC and will operate as part of ICIG’s pharmaceutical business within the Corden Pharma platform. The companies’ goal is to close the transaction during the first quarter of 2011. Financial terms are not disclosed.

