We serve 2,2,2-Trifluoroethylamine CAS:753-90-2 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
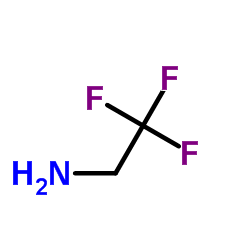
Contact us for information like 2,2,2-Trifluoroethylamine chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,Trifluoroethylamine physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,2,2,2-Trifluoroethylamine Use and application,Trifluoroethylamine technical grade,usp/ep/jp grade.
Related News: Countries across Asia, Europe, Australia and North America have also had confirmed cases, including the UK.DL-2-Hydroxybutyric Acid Sodium Salt manufacturer It does not require a production license for the drug substance, and can be produced in an ordinary chemical plant. As long as it reaches a certain level, it can be used for the synthesis of the drug substance.allidochlor supplier It does not require a production license for the drug substance, and can be produced in an ordinary chemical plant. As long as it reaches a certain level, it can be used for the synthesis of the drug substance.(R)-(+)-2-(4-Hydroxyphenoxy)propionic acid vendor Under the sub-licensing agreement, Mankind will market the drug under its own trademark while Glenmark will manufacture and supply it to the drug firm.This means that the drug attributes of the drug substance will be lost in the future, and the monopoly power of some drug substances will also be lost. The preparation company will become the main person in charge of the drug. The drug preparation company will be responsible for the quality of the original excipients. It will be more cautious, some raw and auxiliary materials companies whose quality cannot be guaranteed will be gradually eliminated, and the industry concentration will be further improved.

