We serve 2,4,5,6-Tetraaminopyrimidine sulfate CAS:5392-28-9 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
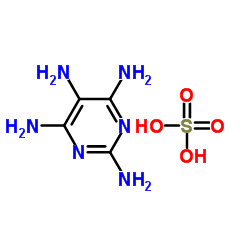
Contact us for information like 2,4,5,6-Tetraaminopyrimidine sulfate chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,2,4,5,6-Tetraaminopyrimidine Sulfate Hydrate physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,Pyrimidine-2,4,5,6-tetraamine sulfate Use and application,2,4,5,6-Tetraaminopyrimidine Sulfate Hydrate technical grade,usp/ep/jp grade.
Related News: Many domestic raw material pharmaceutical and intermediate manufacturing companies with strong capital and talent reserves and advanced technology and processes have developed in the country, and the competitiveness of market participants has continued to increase.5-Formyl-2,3-dihydro-1-[3-(phenylmethoxy)propyl]-1H-indole-7-carbonitrile manufacturer Pre-approval Access Programs (also known as expanded access, early access, compassionate use, named patient supply) are regulatory-compliant processes permitting experimental agents in development to be made available upon the request of a physician or a patient for appropriate patients for whom no alternative treatment option exists in their country.phenoxyacetic acid supplier Pre-approval Access Programs (also known as expanded access, early access, compassionate use, named patient supply) are regulatory-compliant processes permitting experimental agents in development to be made available upon the request of a physician or a patient for appropriate patients for whom no alternative treatment option exists in their country.2-pyrazin-2-ylethanethiol vendor Citizens and residents will be allowed entry to New Zealand, but will be required to quarantine themselves for 14 days, Prime Minister Jacinda Ardern said.The Company’s immuno-oncology product candidates include natural killer (NK) cell and T-cell cancer immunotherapies, which are designed to synergize with well-established cancer therapies, including immune checkpoint inhibitors and monoclonal antibodies, and to target tumor-associated antigens with chimeric antigen receptors (CARs).

