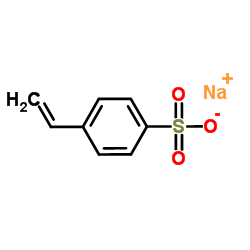
We serve sodium,4-ethenylbenzenesulfonate CAS:2695-37-6 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
| SPECIFICATION PROPERTIES | STANDARD | RESULTS |
| Appearance | Free flowing granules or powder without visible contamination | |
| Vinyl Activity(as purity of sss, by dry basis),% | 89-100 | 90.8 |
| Water,% | 8-12 | 11.4 |
| Color,(1%APHA)(HaZen) | ≤50 | 17 |
| pH(10%aqueous solution) | 7.5-11 | 9.7 |
| Filterable Matter, % | ≤0.06 | 0.01 |
| Sodium Sulfate,% | ≤0.8 | 0.26 |
| Halides,% | ≤6 | 0.9 |
| Light Absorbancc/cm at 600nm | ≤0.035 | 0.01 |
| Iron,ppm | ≤15 | 2 |
Contact us for information like sodium,4-ethenylbenzenesulfonate chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,Sodium 4-vinylbenzenesulfonate physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,Sodium 4-vinylbenzenesulphonate Use and application,4-Vinylbenzenesulfonic Acid Sodium Salt Hydrate technical grade,usp/ep/jp grade.
Related News: Nevertheless, many other didn’t appear to need confirmation. Posts on Weibo, China’s Twitter-like platform, purportedly showed people lining up at night outside pharmacies across China to buy Shuanghuanglian. 2-(4-(6-(2,5-dimethyl-1H-pyrrol-1-yl)pyridin-2-yl)phenyl)acetic acid manufacturer Higher-risk MDS is a disease with significant unmet need, and we are pleased to be able to support healthcare professionals seeking access to rigosertib, ahead of its commercial launch,” said Mark Corbett, EVP, Inceptua Medicines Access.2-((4,6-bis(bis(2-hydroxyethyl)amino)-1,3,5-triazin-2-yl)amino)benzoic acid supplier Our quality control staff then conducts analyses in the testing laboratory to examine whether the API manufactured is ultrapure. 4-Hydrazinobenzoic acid vendor High-barrier generic drugs: Usually for the production of drugs whose patents have just expired or are about to expire, the market is only competed by original research and a few generic drug companies.INSPIRE is a global, multi-center, randomized, controlled study to assess the efficacy and safety of IV rigosertib in higher-risk MDS (HR-MDS) patients who had progressed on, failed to respond to, or relapsed after previous treatment with a hypomethylating agent (HMA) within nine cycles over the course of one year after initiation of HMA treatment.

