We serve Calcium iodide tetrahydrate CAS:13640-62-5 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
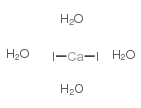
Boiling point: 1100ºC
| Items of Analysis | Standard of Analysis | Test Results |
| Appearance | White to light yellow crystalline powder | Conform |
| Sulfate | ≤0.1% | 0.10% |
| Chloride | ≤0.5% | <0.5% |
| Assay( as I) | ≥68.5% | 68.90% |
| CaI2·4H2O Assay | ≥99.0% | 99.30% |
| Conclusion | Conforms to Factory Standard | |
Contact us for information like Calcium iodide tetrahydrate chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,calcium,diiodide,tetrahydrate physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,Calcium iodide tetrahydrate Use and application,Calcium iodide tetrahydrate technical grade,usp/ep/jp grade.
Related News: Singapore, Australia, New Zealand, and the United States have banned all foreign nationals traveling from China from entering the country.2-[2-[2-(dimethylamino)ethyl-methylamino]ethyl]isoindole-1,3-dione manufacturer Singapore, Australia, New Zealand, and the United States have banned all foreign nationals traveling from China from entering the country.decyl 2-methylprop-2-enoate supplier In 2016, in order to promote the innovative development, transformation and upgrading of the pharmaceutical industry, the Development and Reform Commission led the compilation of the “Guiding Opinions on Promoting the Healthy Development of the Pharmaceutical Industry”, which put forward requirements for all aspects of the medical industry and specifically proposed support for the field of chemical raw materials. .S-2-Benzothiazolyl (Z)-2-(5-amino-1,2,4-thladlazol-3-yl)-2-MethoxylMino thioacetate vendor In 2016, in order to promote the innovative development, transformation and upgrading of the pharmaceutical industry, the Development and Reform Commission led the compilation of the “Guiding Opinions on Promoting the Healthy Development of the Pharmaceutical Industry”, which put forward requirements for all aspects of the medical industry and specifically proposed support for the field of chemical raw materials. .In 2016, in order to promote the innovative development, transformation and upgrading of the pharmaceutical industry, the Development and Reform Commission led the compilation of the “Guiding Opinions on Promoting the Healthy Development of the Pharmaceutical Industry”, which put forward requirements for all aspects of the medical industry and specifically proposed support for the field of chemical raw materials. .

