We serve Chemical Name:sulfallate CAS:95-06-7 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
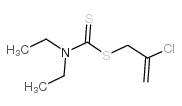
Chemical Name:sulfallate
CAS.NO:95-06-7
Synonyms:CDEC;Vegadex;Chlorallyl diethyldithiocarbamate;Vegedex;Diaethyl-dithiocarbamidsaeure-(2-chlor-allylester);2-chloroprop-2-enyl N,N-diethylcarbamodithioate;Caswell No. 180;2-Chloroallyl diethyldithiocarbamate;2-chloroallyl N,N-diethyldithiocarbamate;Thioallate;2-chloroallyl diethyl(dithiocarbamate);2-chloro-2-propen-1-yl N,N-diethylcarbamodithioate;EINECS 205-701-7;MFCD00048530;2-chloroprop-2-en-1-yl diethylcarbamodithioate;diethyl-dithiocarbamic acid-(2-chloro-allyl ester);SULFALLATE;Vegadex super
Molecular Formula:C8H14ClNS2
Molecular Weight:223.78600
HS Code:2930200016
Physical and Chemical Properties:
Melting point:N/A
Boiling point:264.3ºC at 760mmHg
Density:1.159g/cm3
Index of Refraction:1.561
PSA:60.63000
Exact Mass:223.02600
LogP:3.09880
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:UN3082 9/PG 3
Packing Group:
Contact us for information like CDEC chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,Vegadex super physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,SULFALLATE Use and application,Diaethyl-dithiocarbamidsaeure-(2-chlor-allylester) technical grade,usp/ep/jp grade.
Related News: Under the plan, nonessential hospital staff members who belong to the union would not go to work on Monday. sulfallate manufacturer ��Administrative Measures for the Joint Review, Review and Approval of Raw Materials, Medicinal Auxiliaries and Pharmaceutical Packaging Materials and Pharmaceutical Preparations�� (Consultation Draft) issued by the State Food and Drug Administration in December 2017. Supervision departments no longer accept applications for registration of APIs, pharmaceutical excipients, and packaging materials. sulfallate supplier As you can see, a considerable number of staff is involved in the production phase until an API is finally manufactured. sulfallate vendor ��Administrative Measures for the Joint Review, Review and Approval of Raw Materials, Medicinal Auxiliaries and Pharmaceutical Packaging Materials and Pharmaceutical Preparations�� (Consultation Draft) issued by the State Food and Drug Administration in December 2017. Supervision departments no longer accept applications for registration of APIs, pharmaceutical excipients, and packaging materials. sulfallate factory ��Administrative Measures for the Joint Review, Review and Approval of Raw Materials, Medicinal Auxiliaries and Pharmaceutical Packaging Materials and Pharmaceutical Preparations�� (Consultation Draft) issued by the State Food and Drug Administration in December 2017. Supervision departments no longer accept applications for registration of APIs, pharmaceutical excipients, and packaging materials.

