We serve Chemical Name:2-Chloro-4-ethoxymethyl-pyrimidine CAS:1289385-59-6 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
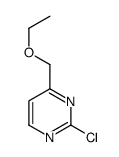
Chemical Name:2-Chloro-4-ethoxymethyl-pyrimidine
CAS.NO:1289385-59-6
Synonyms:2-Chloro-4-ethoxymethylpyrimidine
Molecular Formula:C7H9ClN2O
Molecular Weight:172.61200
HS Code:
Physical and Chemical Properties:
Melting point:N/A
Boiling point:275℃
Density:1.202
Index of Refraction:
PSA:35.01000
Exact Mass:172.04000
LogP:1.66650
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like 2-Chloro-4-ethoxymethylpyrimidine chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,2-Chloro-4-ethoxymethylpyrimidine physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,2-Chloro-4-ethoxymethylpyrimidine Use and application,2-Chloro-4-ethoxymethylpyrimidine technical grade,usp/ep/jp grade.
Related News: The company reported that it was cooperating with the Justice Department probe and that an outside law firm was also “investigating these allegations thoroughly.” 2-Chloro-4-ethoxymethyl-pyrimidine manufacturer An active pharmaceutical ingredient (API) is a substance, or a mixture of ingredients, combined in the manufacture of a pharmaceutical or drug. 2-Chloro-4-ethoxymethyl-pyrimidine supplier The company reported that it was cooperating with the Justice Department probe and that an outside law firm was also “investigating these allegations thoroughly.” 2-Chloro-4-ethoxymethyl-pyrimidine vendor Bulk drug substances mainly include vitamins, antibiotics, hormones, antipyretics and analgesics, among which vitamin C, erythromycin thiocyanate, penicillin, azithromycin, and semi-synthetic cephalosporins are the most concentrated products in the industry in terms of production and overcapacity. 2-Chloro-4-ethoxymethyl-pyrimidine factory In April 2019, Glenmark had received approval from the Drugs Controller General of India (DCGI) for Remogliflozin Etabonate after successfully completing Phase-3 clinical trials.

