We serve Chemical Name:4-(2-Bromoethyl)benzonitrile CAS:72054-56-9 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
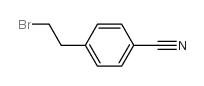
Chemical Name:4-(2-Bromoethyl)benzonitrile
CAS.NO:72054-56-9
Synonyms:(4-cyanophenethyl)bromide;4-bromoethyl benzonitrile;2-(4-cyanophenyl)ethyl bromide;Benzonitrile,4-(2-bromoethyl);4-(2-Brom-aethyl)-benzonitril;4-(2-bromo-ethyl)-benzonitrile
Molecular Formula:C9H8BrN
Molecular Weight:210.07100
HS Code:2926909090
Physical and Chemical Properties:
Melting point:N/A
Boiling point:290.363ºC at 760 mmHg
Density:1.437g/cm3
Index of Refraction:1.575
PSA:23.79000
Exact Mass:208.98400
LogP:2.49568
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like (4-cyanophenethyl)bromide chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,4-(2-bromo-ethyl)-benzonitrile physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,(4-cyanophenethyl)bromide Use and application,Benzonitrile,4-(2-bromoethyl) technical grade,usp/ep/jp grade.
Related News: The drug review center will establish a registration platform and database for APIs, pharmaceutical excipients and pharmaceutical packaging materials, which means that the domestic drug substance DMF system is expected to be gradually implemented. 4-(2-Bromoethyl)benzonitrile manufacturer Raw material drug manufacturing is listed as one of the top ten key remediation industries, and it is necessary to implement clean transformation, new construction, reconstruction, and expansion of the ten key remediation industry construction projects to implement equal or reduced emissions of major pollutants; ) The industry implements the green enzyme production technology transformation; at the same time, the ten articles of water classify the industrial discharged sewage, and the sewage discharge of API companies is restricted. 4-(2-Bromoethyl)benzonitrile supplier We found over half the products we tested contained elevated fluorine levels,” Bruton said.
The cosmetic categories that had the highest percentage of 213 high fluorine products were foundations (63%), eye products (58%), mascaras (47%), and lip products (55%), the study found.
Even more concerning was that cosmetics containing high levels of fluorine more often than not failed to disclose any PFAS chemicals on their labels, Bruton noted.
Further analysis of 29 cosmetics with high fluorine levels revealed that they contained between four and 13 specific PFAS chemicals, researchers found. However, only 1 of the 29 products listed PFAS as an ingredient on the product label.
“Even if a consumer is doing their due diligence and trying to avoid harmful chemicals by reading labels, our work is showing that these harmful chemicals are often not disclosed,” Bruton said.
Despite this, Bruton recommends that consumers who want to limit their exposure to PFAS read the labels anyway, to at least avoid products where the chemicals are accurately listed.
High levels of fluorine were frequently found in products advertised as “long-lasting” and “wear-resistant,” which could provide another clue for discerning consumers.
But in the end, there’s not much consumers can do to solve the problem.
“It’s important that the government step up to regulate ingredients in cosmetics with more stringency,” Bruton said. “It’s also time the cosmetics industry steps up and begins efforts to move away from this class of chemicals. 4-(2-Bromoethyl)benzonitrile vendor The drug review center will establish a registration platform and database for APIs, pharmaceutical excipients and pharmaceutical packaging materials, which means that the domestic drug substance DMF system is expected to be gradually implemented. 4-(2-Bromoethyl)benzonitrile factory The FDA issued a stark warning to the public urging them to stop using rapid COVID-19 antigen tests developed by Innova Medical Group, the company previously tapped by the U.K. government for hundreds of millions of kits to help regularly screen the country’s population.
The cosmetic categories that had the highest percentage of 213 high fluorine products were foundations (63%), eye products (58%), mascaras (47%), and lip products (55%), the study found.
Even more concerning was that cosmetics containing high levels of fluorine more often than not failed to disclose any PFAS chemicals on their labels, Bruton noted.
Further analysis of 29 cosmetics with high fluorine levels revealed that they contained between four and 13 specific PFAS chemicals, researchers found. However, only 1 of the 29 products listed PFAS as an ingredient on the product label.
“Even if a consumer is doing their due diligence and trying to avoid harmful chemicals by reading labels, our work is showing that these harmful chemicals are often not disclosed,” Bruton said.
Despite this, Bruton recommends that consumers who want to limit their exposure to PFAS read the labels anyway, to at least avoid products where the chemicals are accurately listed.
High levels of fluorine were frequently found in products advertised as “long-lasting” and “wear-resistant,” which could provide another clue for discerning consumers.
But in the end, there’s not much consumers can do to solve the problem.
“It’s important that the government step up to regulate ingredients in cosmetics with more stringency,” Bruton said. “It’s also time the cosmetics industry steps up and begins efforts to move away from this class of chemicals. 4-(2-Bromoethyl)benzonitrile vendor The drug review center will establish a registration platform and database for APIs, pharmaceutical excipients and pharmaceutical packaging materials, which means that the domestic drug substance DMF system is expected to be gradually implemented. 4-(2-Bromoethyl)benzonitrile factory The FDA issued a stark warning to the public urging them to stop using rapid COVID-19 antigen tests developed by Innova Medical Group, the company previously tapped by the U.K. government for hundreds of millions of kits to help regularly screen the country’s population.

