We serve 2′-Bromo-4′-fluoroacetophenone CAS:1006-39-9 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
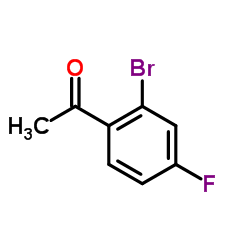
Contact us for information like 2′-Bromo-4′-fluoroacetophenone chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,2′-BROMO-4′-FLUOROACETOPHENONE physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,2′-Bromo-4′-fluoroacetophenone Use and application,1-(2-Bromo-4-fluorophenyl)ethanone technical grade,usp/ep/jp grade.
Related News: Active Pharmaceutical Ingredients (APIs): Pharmaceutical active ingredients, which are the basic substances that constitute the pharmacological effects of pharmaceuticals, and are prepared by chemical synthesis, plant extraction, or biotechnology.HC VIOLET NO. 2 manufacturer US citizens who’ve been in other parts of mainland China in the last 14 days will undergo screening at US ports of entry and up to 14 days of self-monitoring.Furfural supplier The iLet is designed to function as three medical devices in one. It can be configured as an insulin-only bionic pancreas, a glucagon-only bionic pancreas, or a bihormonal bionic pancreas using insulin and glucagon.1,4-Dibromobutane vendor In 2016, in order to promote the innovative development, transformation and upgrading of the pharmaceutical industry, the Development and Reform Commission led the compilation of the “Guiding Opinions on Promoting the Healthy Development of the Pharmaceutical Industry”, which put forward requirements for all aspects of the medical industry and specifically proposed support for the field of chemical raw materials. .The foundational patent, which expires in 2034, is owned by MSK and is licensed exclusively to Fate Therapeutics for all human therapeutic uses.

