We serve 2-Chloro-3-fluorobenzaldehyde CAS:96516-31-3 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
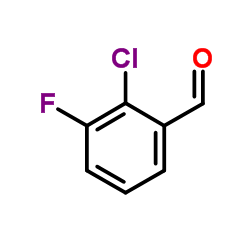
Contact us for information like 2-Chloro-3-fluorobenzaldehyde chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,2-Chloro-3-fluorobenzaldehyde physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,2-Chloro-3-fluorobenzaldehyde Use and application,2-Chloro-3-fluorobenzaldehyde technical grade,usp/ep/jp grade.
Related News: But the ban from Italy remains, Joseph Wu, Taiwan’s foreign minister, said on Sunday.6-Bromo-5-chloropyridin-3-amine manufacturer Bulk bulk medicines: overcapacity, low prices, future capacity and output will be reduced, and it is possible to transfer foreign production.N-Ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline supplier ViiV, in which Pfizer and Shionogi have small stakes, said it received a so-called complete response letter (CRL) from the FDA in which the regulator questioned the treatment’s chemistry, manufacturing and controls process, but not its safety.Ethyl 2-Sulfamoylbenzoate vendor ViiV, in which Pfizer and Shionogi have small stakes, said it received a so-called complete response letter (CRL) from the FDA in which the regulator questioned the treatment’s chemistry, manufacturing and controls process, but not its safety.ViiV, in which Pfizer and Shionogi have small stakes, said it received a so-called complete response letter (CRL) from the FDA in which the regulator questioned the treatment’s chemistry, manufacturing and controls process, but not its safety.

