We serve 2-(TRIFLUOROMETHOXY)BENZOYL CHLORIDE CAS:162046-61-9 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
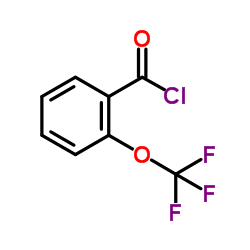
Contact us for information like 2-(TRIFLUOROMETHOXY)BENZOYL CHLORIDE chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,o-trifluoromethoxybenzoyl chloride physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,Trifluoromethoxybenzoyl chloride Use and application,Trifluoromethoxybenzoyl chloride technical grade,usp/ep/jp grade.
Related News: If the drug is in a syrup form, then the excipient is the liquid that has been used to make it as such.2-((2-Nitro-4-(trifluoromethyl)phenyl)amino)ethanol manufacturer If the drug is in a syrup form, then the excipient is the liquid that has been used to make it as such.1,12-Dodecanediol supplier If the drug is in a syrup form, then the excipient is the liquid that has been used to make it as such.3-bromo-9-naphthalen-2-ylcarbazole vendor If the drug is in a syrup form, then the excipient is the liquid that has been used to make it as such.Pre-approval Access Programs (also known as expanded access, early access, compassionate use, named patient supply) are regulatory-compliant processes permitting experimental agents in development to be made available upon the request of a physician or a patient for appropriate patients for whom no alternative treatment option exists in their country.

