We serve Tetramethylpyrazine CAS:1124-11-4 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
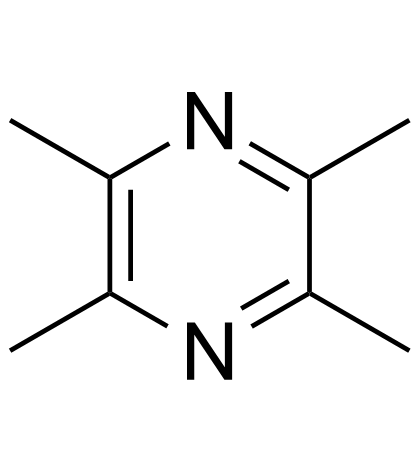
Contact us for information like Tetramethylpyrazine chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,2,3,5,6-tetramethylpyrazine physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,Tetramethylpyrazine Use and application,Pyrazine, tetramethyl- technical grade,usp/ep/jp grade.
Related News: The drug, Imfinzi, when added to chemotherapy and the drugmaker’s other cancer drug tremelimumab, significantly improved the survival of patients without the disease progressing, when compared to chemotherapy alone, the company said.[4-(4-fluorophenyl)-2-[methyl(methylsulfonyl)amino]-6-propan-2-ylpyrimidin-5-yl]methyl-triphenylphosphanium,bromide manufacturer The drug, Imfinzi, when added to chemotherapy and the drugmaker’s other cancer drug tremelimumab, significantly improved the survival of patients without the disease progressing, when compared to chemotherapy alone, the company said.2-Bromoethyl acetate supplier At present, API companies basically use multi-functional and multi-purpose workshops, and the main equipment uses multi-functional reactors to achieve flexible utilization of production capacity.2-imidazol-1-ylacetic acid vendor The clinical trial International Study of Phase 3 IV RigosErtib, or INSPIRE, was finalized following guidance received from the U.S. Food and Drug Administration and European Medicines Agency.The clinical trial International Study of Phase 3 IV RigosErtib, or INSPIRE, was finalized following guidance received from the U.S. Food and Drug Administration and European Medicines Agency.

