We serve Chemical Name:triisobutylphosphine CAS:4125-25-1 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
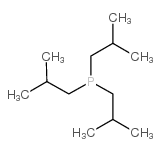
Chemical Name:triisobutylphosphine
CAS.NO:4125-25-1
Synonyms:tri-isobutylphosphane;EINECS 223-934-2;Tri-i-butylphosphine;Triisobutylphosphine;MFCD00015049
Molecular Formula:C12H27P
Molecular Weight:202.31700
HS Code:2931900090
Physical and Chemical Properties:
Melting point:N/A
Boiling point:126ºC50 mm Hg(lit.)
Density:0.811 g/mL at 25ºC(lit.)
Index of Refraction:n20/D 1.451(lit.)
PSA:13.59000
Exact Mass:202.18500
LogP:4.43630
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:UN 2845 4
Packing Group:
Contact us for information like tri-isobutylphosphane chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,MFCD00015049 physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,tri-isobutylphosphane Use and application,Tri-i-butylphosphine technical grade,usp/ep/jp grade.
Related News: The move follows a Class I recall, the FDA’s most serious, launched by Innova in late April amid “significant concerns” about the test’s accuracy—and alongside an official warning letter delivered to the company this week. triisobutylphosphine manufacturer An FDA inspection also turned up a long list of sanitary problems and bad manufacturing practices at the Emergent plant. triisobutylphosphine supplier Featured APIs: Production capacity continues to expand, targeted patents expire. Original research drugs: industry entry barriers are high, performance stability and certainty are high. triisobutylphosphine vendor Featured APIs: Production capacity continues to expand, targeted patents expire. Original research drugs: industry entry barriers are high, performance stability and certainty are high. triisobutylphosphine factory The move follows a Class I recall, the FDA’s most serious, launched by Innova in late April amid “significant concerns” about the test’s accuracy—and alongside an official warning letter delivered to the company this week.

