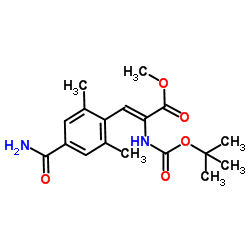
We serve (Z)-methyl 2-((tert-butoxycarbonyl)amino)-3-(4-carbamoyl-2,6-dimethylphenyl)acrylate CAS:864825-84-3 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
Contact us for information like (Z)-methyl 2-((tert-butoxycarbonyl)amino)-3-(4-carbamoyl-2,6-dimethylphenyl)acrylate chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,Methyl (2Z)-3-(4-carbamoyl-2,6-dimethylphenyl)-2-({[(2-methyl-2-propanyl)oxy]carbonyl}amino)acrylate physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,(Z)-methyl 3-(4-carbamoyl-2,6-dimethylphenyl)-2-(Boc-amino)acrylate; Use and application,(Z)-methyl 2-((tert-butoxycarbonyl)amino)-3-(4-carbamoyl-2,6-dimethylphenyl)acrylate technical grade,usp/ep/jp grade.
Related News: Myelodysplastic syndromes (MDS) are conditions that can occur when the blood-forming cells in the bone marrow become dysfunctional and thus produce an inadequate number of circulating blood cells.1-Mercaptooctane manufacturer More than 2,000 new cases were also recorded in the country in the past 24 hours, raising the worldwide total to nearly 14,380, according to Chinese and World Health Organization data. The vast majority of the cases are inside China; about 100 cases have been confirmed in at least 23 other countries.3-hydroxy-N-(4-methoxyphenyl)naphthalene-2-carboxamide supplier APIs are generally manufactured through a variety of processes that include.3-fluoro-1-oxidopyridin-1-ium vendor From a global perspective, China’s API companies have also performed well. According to the report of the American Transparent Medicine website, in 2016 the top ten pharmaceutical companies in the global API market, Chinese pharmaceutical companies accounted for 6 seats.Oligomannate, which uses extract from marine brown algae as raw material, received a conditional green light to treat mild-to-moderate level AD, the National Medical Products Administration (NMPA) said in a statement on its website late on Saturday.

