We serve N,O-Di(2-hydroxyethyl)-2-amino-5-nitrophenol CAS:59820-43-8 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
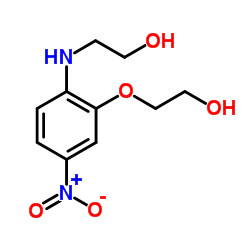
Contact us for information like N,O-Di(2-hydroxyethyl)-2-amino-5-nitrophenol chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,HC yellow 4 physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,N,O-Di(2-hydroxyethyl)-2-amino-5-nitrophenol Use and application,N,O-Di(2-hydroxyethyl)-2-amino-5-nitrophenol technical grade,usp/ep/jp grade.
Related News: Countries across Asia, Europe, Australia and North America have also had confirmed cases, including the UK.3-Bromo-2-methoxypyridine manufacturer If the government does not close the border and heed their other demands by 9 p.m., union members handling emergency services would also strike, the union said.Furfural supplier From the perspective of the corresponding formulation manufacturer, the drug substance needs to meet the requirements of impurities and stability. The production base must pass the international quality system certifications such as cGMP and EuGMP. At the same time, the drug substance company must have sufficient capacity.cis-11-Eicosenoic acid vendor From the perspective of the corresponding formulation manufacturer, the drug substance needs to meet the requirements of impurities and stability. The production base must pass the international quality system certifications such as cGMP and EuGMP. At the same time, the drug substance company must have sufficient capacity.”This is particularly important in reducing overall disease burden. Moreover, this strategic decision will not only strengthen our diabetes portfolio but also help consolidate our position as the fastest-growing player in the anti-diabetes segment,” Mankind Pharma Director of Marketing Sanjay Koul said in a statement.

