We serve L-Histidine Monohydrochloride Monohydrate CAS:5934-29-2 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
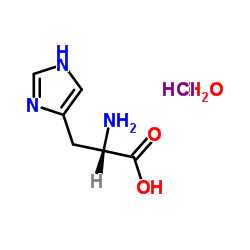
L-Histidine Monohydrochloride Monohydrate
A monohydrochloride monohydrate salt of L-histidine. L-Histidine is an essential amino acid. It is widely used as an ingredient in infusion.
| Cas.No | 5934-29-2 |
| Assay | 99.0-101.0% |
| Specification | White crystals or crystalline powder, odorless, slightly bitter acid taste |
| Applications | Infusion, Cell culture media, Biotech |
| Packaging | 25kg&50kg |
| Pharmacopeia | EP,FCC |
| STORAGE | Controlled room temperature in tight container |
SPECIFICATION AND PROCEDURE
| State of solution (Transmittance) |
Not Less Than 98.0% |
| pH | 3.5~4.5 |
| Specific rotation[α]20D | +8.9~+9.5° |
| Ammonium (NH4) | Not More Than 0.020% |
| Chloride content (Cl) | 16.6~17.1% |
| Sulfate (SO4) | Not More Than 0.020% |
| Iron (Fe) | Not More Than 10 ppm |
| Heavy metals (Pb)** | Not More Than 10 ppm |
| Arsenic (As2O3) | Not More Than 1 ppm |
| WATER | 7.2 to 10.0% |
| Residue on ignition | Not More Than 0.10% |
| Related substances | Not More Than 0.2% |
| Endotoxin* | Less Than 6.0 EU/g |
| Assay (dry basis) | 99.0~101.0% |
· * The endotoxin-certified grade will be supplied on request.
· ** FCC grade (Lead : Not More Than 5 mg/kg) will be supplied on request.
· This product meets requirements of residual solvents listed in the current JP, USP and EP.
Contact us for information like L-Histidine Monohydrochloride Monohydrate chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,(2S)-2-amino-3-(1H-imidazol-5-yl)propanoic acid,hydrate,hydrochloride physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,L-Histidine, monohydrochloride, monohydrate Use and application,(2S)-2-amino-3-(1H-imidazol-5-yl)propanoic acid,hydrate,hydrochloride technical grade,usp/ep/jp grade.
Related News: Steven M. Fruchtman, M.D., President and Chief Executive Officer of Onconova, added “The rigosertib Pre-approval Access Program is a key strategic initiative for Onconova.(4R,6R)-tert-Butyl-6-(2-aminoethyl)-2,2-dimethyl-1,3-dioxane-4-acetate manufacturer In addition, a high-standard GMP workshop is under construction and is expected to be put into production within the year. As the capacity bottleneck is broken, the pharmaceutical intermediate business is expected to grow rapidly in the future, providing the company with new growth momentum.1-Phenyl-1,2-dihydro-3H-1,2,4-triazol-3-one supplier After this long manufacturing process, it is purified until it reaches a very high degree of purity and finally becomes an API.(R)-3-Cyclopentyl-3-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazol-1-yl)propanenitrile vendor After this long manufacturing process, it is purified until it reaches a very high degree of purity and finally becomes an API.Refers to the active pharmaceutical ingredients used in the manufacture of original research drugs (patent drugs or innovative drugs). It mainly meets the needs of international original research drug companies and emerging biopharmaceutical companies for innovative drugs at various stages of clinical research, registration approval and commercialization of drugs. Contains advanced intermediates used in the manufacture of this drug substance that need to be regulated by regulatory authorities.

