We serve DL-Butyrine CAS:2835-81-6 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
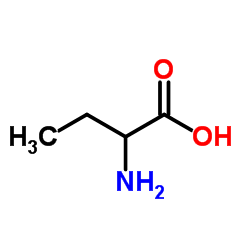
Chemical Name: α-aminobutyric acid
CAS.NO:2835-81-6
Synonyms:
DL-Butyrine
DL-2-Aminobutyric Acid
Butanoic acid, 2-amino-, (±)-
2-Aminobutyric Acid
2-aminobutanoic acid
alpha-aminobutyric acid
DL-Butyrine-d3
DL-2-aminobutanoic acid
H-DL-ABU-OH
homoalanine
2-aminobutanoic
DL-.α.-Amino-n-butyric acid (.β.-form)
DL-Ethylglycine
2-amino-n-butyric acid
Molecular Formula: C4H9NO2
Molecular Weight:103.12000
Physical and Chemical Properties:
Density: 1.105 g / cm3
Boiling point: 215.2ºC at 760 mmHg
Melting point: 291-293ºC (dec.)
Flash point: 83.9ºC
Refractive index: 1.438
Specification:
Appearance:White crystalline powder
Purity:≥98%
mpurity: 0.01%
Packing:
25kg cardboard drum or according to customer specified requirements
Storage:Stored in a cool and dry well-closed container. Keep away from moisture and strong light/heat.
Application:
Intermediates of Levetiracetam CAS:102767-28-2
Contact us for information like α-aminobutyric acid chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,2-amino-n-butyric acid physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,homoalanine Use and application,α-aminobutyric acid technical grade,usp/ep/jp grade.
Related News: Social media users — including numerous medical experts — questioned whether the findings were supported by clinical evidence from treating coronavirus patients.1,3,5,7-tetramethylcyclotetrasiloxane manufacturer If the government does not close the border and heed their other demands by 9 p.m., union members handling emergency services would also strike, the union said.2'-O-methylcytidine supplier From the perspective of the corresponding formulation manufacturer, the drug substance needs to meet the requirements of impurities and stability. The production base must pass the international quality system certifications such as cGMP and EuGMP. At the same time, the drug substance company must have sufficient capacity.1-bromo-5-iodopentane vendor Onconova is currently in the clinical development stage with oral and IV rigosertib, including clinical trials studying single agent IV rigosertib in second-line higher-risk MDS patients (pivotal Phase 3 INSPIRE trial) and oral rigosertib plus azacitidine in first-line and refractory higher-risk MDS patients (Phase 2).Since first being reported in the city of Wuhan, where it is believed to have originated at a seafood market, the virus has not provoked unusual symptoms in people who have been diagnosed.

