We serve 2′-bromo-2-iodobiphenyl CAS:39655-12-4 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
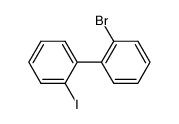
Contact us for information like 2′-bromo-2-iodobiphenyl chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,2,2′-BIBP physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,2-iodo-2′-bromobiphenyl Use and application,2′-Bromo-2-iodo-biphenyl technical grade,usp/ep/jp grade.
Related News: Any drug or medication is composed of two components.2-(2-Chloroethoxy)-Benzenesulfonamide manufacturer Any drug or medication is composed of two components.4-chloro-2,3-dihydroisoindol-1-one supplier It is foreseeable that in the future, the capacity and output of bulk APIs in China will decrease, the supply-demand relationship will be balanced, and prices and profits will gradually return to a more reasonable range. The era of low prices in the past will be gone forever. Individual APIs Varieties may even lose their price competitive advantage and move abroad.Methyl 4-bromobenzoate vendor The CRL for the Cabenuva injection, containing two active ingredients cabotegravir and Janssen’s rilpivirine, follows U.S. market approval in April for its once-a-day pill Dovato, also a two-drug combination.The CRL for the Cabenuva injection, containing two active ingredients cabotegravir and Janssen’s rilpivirine, follows U.S. market approval in April for its once-a-day pill Dovato, also a two-drug combination.

