We serve Chemical Name:2-Chloro-6-fluoroquinazolin-4(3H)-one CAS:769158-12-5 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
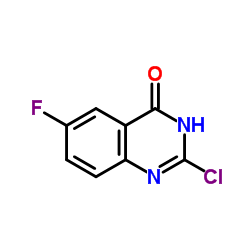
Chemical Name:2-Chloro-6-fluoroquinazolin-4(3H)-one
CAS.NO:769158-12-5
Synonyms:2-chloro-6-fluoroquinazolin-4(3H)-one;2-chloro-6-fluoro-3H-quinazolin-4-one;2-chloro-6-fluoroquinazolin;4(3H)-Quinazolinone, 2-chloro-6-fluoro-;2-Chloro-6-fluoro-4(1H)-quinazolinone
Molecular Formula:C8H4ClFN2O
Molecular Weight:198.582
HS Code:2933990090
Physical and Chemical Properties:
Melting point:N/A
Boiling point:339.8ºC at 760 mmHg
Density:1.6±0.1 g/cm3
Index of Refraction:1.665
PSA:45.75000
Exact Mass:197.999619
LogP:1.77
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like 2-chloro-6-fluoroquinazolin-4(3H)-one chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,2-Chloro-6-fluoro-4(1H)-quinazolinone physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,2-chloro-6-fluoroquinazolin Use and application,2-Chloro-6-fluoro-4(1H)-quinazolinone technical grade,usp/ep/jp grade.
Related News: Rigosertib, Onconova��s lead candidate, is a proprietary Phase 3 small molecule. 2-Chloro-6-fluoroquinazolin-4(3H)-one manufacturer As Reuters reported last month, Lilly tapped the Washington D.C. law firm Covington & Burling LLP to investigate the alleged alterations, which the employees said were meant to downplay serious quality control problems at the plant producing the drugmaker’s COVID-19 antibody treatment. 2-Chloro-6-fluoroquinazolin-4(3H)-one supplier With the improvement of domestic GMP management level, the increase of process development capabilities and international certification experience, China has already possessed the conditions for developing characteristic APIs. 2-Chloro-6-fluoroquinazolin-4(3H)-one vendor This dosage form may also support combination therapy modalities.? To date, over 400 patients have been dosed with the oral formulation of rigosertib in clinical trials.? Combination therapy of oral rigosertib with azacitidine, the standard of care in HR-MDS, has also been studied. Currently, oral rigosertib is being developed as a combination therapy together with azacitidine for patients with higher-risk MDS who require HMA therapy.? 2-Chloro-6-fluoroquinazolin-4(3H)-one factory Rigosertib, Onconova��s lead candidate, is a proprietary Phase 3 small molecule.

