We serve Chemical Name:Fenthoxon Sulfoxide CAS:6552-13-2 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
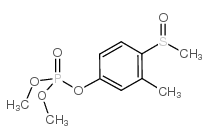
Chemical Name:Fenthoxon Sulfoxide
CAS.NO:6552-13-2
Synonyms:MFCD01632380;dimethyl (3-methyl-4-methylsulfinylphenyl) phosphate
Molecular Formula:C10H15O5PS
Molecular Weight:278.26200
HS Code:2930909090
Physical and Chemical Properties:
Melting point:N/A
Boiling point:N/A
Density:N/A
Index of Refraction:
PSA:90.85000
Exact Mass:278.03800
LogP:3.37780
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:UN 2783
Packing Group:II
Contact us for information like MFCD01632380 chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,dimethyl (3-methyl-4-methylsulfinylphenyl) phosphate physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,MFCD01632380 Use and application,MFCD01632380 technical grade,usp/ep/jp grade.
Related News: The CRL for the Cabenuva injection, containing two active ingredients cabotegravir and Janssen��s rilpivirine, follows U.S. market approval in April for its once-a-day pill Dovato, also a two-drug combination. Fenthoxon Sulfoxide manufacturer Process: The importance of safety is obvious. The research and development of specialty drug substances (especially high-barrier generic drug substance drugs) usually need to avoid the original process patents, and some chemicals are developed because of the complex structure or the harsh synthetic conditions The synthetic route is more difficult, so the importance of process design capabilities of API companies is becoming more important. Fenthoxon Sulfoxide supplier Process: The importance of safety is obvious. The research and development of specialty drug substances (especially high-barrier generic drug substance drugs) usually need to avoid the original process patents, and some chemicals are developed because of the complex structure or the harsh synthetic conditions The synthetic route is more difficult, so the importance of process design capabilities of API companies is becoming more important. Fenthoxon Sulfoxide vendor The cell product candidate is being developed under a collaboration with Memorial Sloan Kettering Cancer Center (MSK) led by Michel Sadelain, MD, PhD. Fenthoxon Sulfoxide factory Process: The importance of safety is obvious. The research and development of specialty drug substances (especially high-barrier generic drug substance drugs) usually need to avoid the original process patents, and some chemicals are developed because of the complex structure or the harsh synthetic conditions The synthetic route is more difficult, so the importance of process design capabilities of API companies is becoming more important.

