We serve Chemical Name:2,3,5,7a-Tetrahydro-1H-pyrrolizine CAS:51463-41-3 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
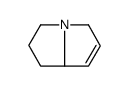
Chemical Name:2,3,5,7a-Tetrahydro-1H-pyrrolizine
CAS.NO:51463-41-3
Synonyms:2,3,5,7a-Tetrahydro-1H-pyrrolizine
Molecular Formula:C7H11N
Molecular Weight:109.16900
HS Code:2933990090
Physical and Chemical Properties:
Melting point:N/A
Boiling point:N/A
Density:N/A
Index of Refraction:
PSA:3.24000
Exact Mass:109.08900
LogP:0.95850
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like 2,3,5,7a-Tetrahydro-1H-pyrrolizine chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,2,3,5,7a-Tetrahydro-1H-pyrrolizine physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,2,3,5,7a-Tetrahydro-1H-pyrrolizine Use and application,2,3,5,7a-Tetrahydro-1H-pyrrolizine technical grade,usp/ep/jp grade.
Related News: Wuhan officials estimate about 5 million people had left the city for the annual Lunar New Year holiday before authorities canceled all outbound flights, trains and buses in an unprecedented lockdown on January 23. 2,3,5,7a-Tetrahydro-1H-pyrrolizine manufacturer he agency said the drugmaker and Emergent must agree that the FDA can share relevant information about the manufacturing of the doses with regulators where the vaccine is shipped. 2,3,5,7a-Tetrahydro-1H-pyrrolizine supplier Professionally produce and submit DMF files, and supplement data at any time during the patent challenge of formulation companies. 2,3,5,7a-Tetrahydro-1H-pyrrolizine vendor Wuhan officials estimate about 5 million people had left the city for the annual Lunar New Year holiday before authorities canceled all outbound flights, trains and buses in an unprecedented lockdown on January 23. 2,3,5,7a-Tetrahydro-1H-pyrrolizine factory Professionally produce and submit DMF files, and supplement data at any time during the patent challenge of formulation companies.

