We serve dodecyl prop-2-enoate CAS:2156-97-0 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
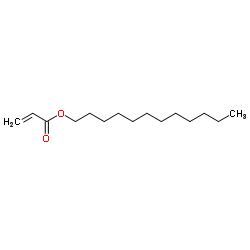
Contact us for information like dodecyl prop-2-enoate chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,n-Dodecyl acrylate physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,Dodecyl Acrylate Use and application,Acrylic acid,dodecyl ester technical grade,usp/ep/jp grade.
Related News: High-barrier generic drugs: Usually for the production of drugs whose patents have just expired or are about to expire, the market is only competed by original research and a few generic drug companies.2-(7-Azabenzotriazole-1-yl)-1,1,3,3-Tetramethyluronium Tetrafluoroborate manufacturer The best you can do is take medicines and treatments for specific symptoms.12-bromododec-1-ene supplier The term active pharmaceutical ingredient may refer to an active chemical within an FDA-regulated drug, or API might mean the entire drug with its active and inactive ingredients.5-Bromopyrimidine vendor With the structural upgrade of the pharmaceutical industry in developed countries, the transfer of related industrial chains has taken place globally, and it has also had a profound impact on Chinese pharmaceutical companies. nearPatents covering oral and injectable rigosertib have been issued in the US and are expected to provide coverage until at least 2037.

