We serve 4,4′-Diacetylbiphenyl CAS:787-69-9 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
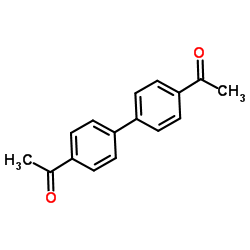
Chemical Name: 4,4'-Diacetylbiphenyl
CAS.NO:787-69-9
Synonyms:1-[4-(4-acetylphenyl)phenyl]ethanone
Molecular Formula: C16H14O2
Molecular Weight:238.28100
Physical and Chemical Properties:
Density: 1.094 g / cm3
Boiling point: 386.5ºC at 760 mmHg
Melting point: 193-195 ° C (lit.)
Flash point: 144.9 ± 17.9 ° C
Refractive index: 1.523
Specification:
Appearance: White crystalline powder
Purity:≥98.0%
Loss on drying:1.0%max
Packing:
25kg/drum. But we could also subpackage it according to our customers' requirements.Such as 1kg/bag, 5kg/bag, 10kg/bag,etc.
Storage:Store in a well-closed container away from moisture.
Application: 4,4'-Diacetylbiphenyl is a useful synthetic intermediate. It can be used to synthesize Daclatasvir which inhibits the HCV protein NS5A, and thus can be used as a drug candidate for the treatment of hepatitis C (HCV).Intermediates of Daclatasvir CAS:1009119-64-5
Contact us for information like 4,4′-Diacetylbiphenyl chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,1-[4-(4-acetylphenyl)phenyl]ethanone physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,4,4′-Diacetylbiphenyl Use and application,4,4′-Diacetylbiphenyl technical grade,usp/ep/jp grade.
Related News: We manufacture APIs and drug manufacturers make medicines from APIs.2-[3,5-bis(trifluoromethyl)phenyl]-2-methylpropanoic acid manufacturer Further research on Oligomannate’s pharmacological mechanism and long-term safety and effectiveness is required, according to the NMPA statement.N2-1[(1S)-Ethoxycarbonyl-3-phenylpropyl]-N6-trifluoroacetyl-L-lysyl-L-proline supplier Further research on Oligomannate’s pharmacological mechanism and long-term safety and effectiveness is required, according to the NMPA statement.methyl 2-sulfanylacetate vendor Further research on Oligomannate’s pharmacological mechanism and long-term safety and effectiveness is required, according to the NMPA statement.Rigosertib, in its intravenous formulation, is currently in Phase 3 clinical development for the treatment of higher-risk MDS.

