We serve Chemical Name:Etocarlide CAS:1234-30-6 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
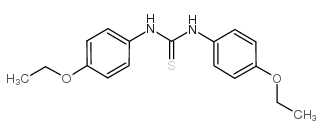
Chemical Name:Etocarlide
CAS.NO:1234-30-6
Synonyms:Ethoxide;Etoksid;Aethoksid;Aethoxyd;4,4′-Diethoxythiocarbanilide;Etocarlide;Aethoxydum;Ethoxyd
Molecular Formula:C17H20N2O2S
Molecular Weight:316.41800
HS Code:2930909090
Physical and Chemical Properties:
Melting point:N/A
Boiling point:446.1ºC at 760mmHg
Density:1.222g/cm3
Index of Refraction:1.655
PSA:74.61000
Exact Mass:316.12500
LogP:4.43890
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like Ethoxide chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,Ethoxyd physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,Aethoxydum Use and application,Etocarlide technical grade,usp/ep/jp grade.
Related News: By drawing these distinctions between APIs and the drugs themselves, manufacturers are able to specialize and pharmacists able to align generic equivalents with brand names. Etocarlide manufacturer ��Administrative Measures for the Joint Review, Review and Approval of Raw Materials, Medicinal Auxiliaries and Pharmaceutical Packaging Materials and Pharmaceutical Preparations�� (Consultation Draft) issued by the State Food and Drug Administration in December 2017. Supervision departments no longer accept applications for registration of APIs, pharmaceutical excipients, and packaging materials. Etocarlide supplier Take penicillin industrial salt and vitamin C as examples. They are the two strategic varieties of chemical raw materials in China, and they are also representatives of severe overcapacity. Etocarlide vendor ��Administrative Measures for the Joint Review, Review and Approval of Raw Materials, Medicinal Auxiliaries and Pharmaceutical Packaging Materials and Pharmaceutical Preparations�� (Consultation Draft) issued by the State Food and Drug Administration in December 2017. Supervision departments no longer accept applications for registration of APIs, pharmaceutical excipients, and packaging materials. Etocarlide factory Citizens and residents will be allowed entry to New Zealand, but will be required to quarantine themselves for 14 days, Prime Minister Jacinda Ardern said.

