We serve Chemical Name:(2H8)Hexanedinitrile CAS:34006-32-1 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
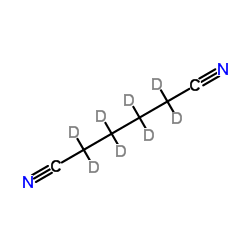
Chemical Name:(2H8)Hexanedinitrile
CAS.NO:34006-32-1
Synonyms:Hexanedinitrile-d8;MFCD00190458;Butanedicarbonitrile-d8;Adipodinitrile-d8;Adipic Dinitrile-d8;Adipic Acid Dinitrile-d8;1,4-Dicyanobutane-d8;(H)Hexanedinitrile;Hexanedinitrile-d;Adiponitrile-d8;Adipic Acid Nitrile-d8
Molecular Formula:C6D8N2
Molecular Weight:116.190
HS Code:
Physical and Chemical Properties:
Melting point:N/A
Boiling point:296.1±13.0 °C at 760 mmHg
Density:0.9±0.1 g/cm3
Index of Refraction:1.432
PSA:47.58000
Exact Mass:116.118965
LogP:-0.32
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:UN 2205 6.1/PG 3
Packing Group:
Contact us for information like Hexanedinitrile-d8 chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,Adipic Acid Nitrile-d8 physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,Butanedicarbonitrile-d8 Use and application,Adipic Dinitrile-d8 technical grade,usp/ep/jp grade.
Related News: Without disclosing or confirming the number of vaccine doses, the FDA said in a news release that it had authorized two batches of the vaccine for use, that several other batches were not suitable for use and that others were being evaluated. (2H8)Hexanedinitrile manufacturer The API can be directly formulated, and the intermediate can only be used to synthesize the next product. Only through the intermediate can the API be manufactured. (2H8)Hexanedinitrile supplier Without disclosing or confirming the number of vaccine doses, the FDA said in a news release that it had authorized two batches of the vaccine for use, that several other batches were not suitable for use and that others were being evaluated. (2H8)Hexanedinitrile vendor In a statement to Fierce Medtech, Innova said it has completed some corrective actions, while others are still underway, and that the U.S. recall was launched to reclaim tests distributed to employees, clinical studies and to customers for early evaluation. The company said it plans to seek an emergency use authorization and comply with all FDA requirements. (2H8)Hexanedinitrile factory Without disclosing or confirming the number of vaccine doses, the FDA said in a news release that it had authorized two batches of the vaccine for use, that several other batches were not suitable for use and that others were being evaluated.

