We serve Ethyl 2-oxo-4-phenylbutyrate CAS:64920-29-2 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
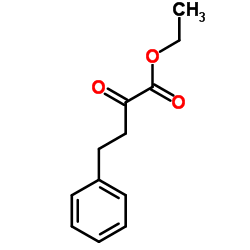
Contact us for information like Ethyl 2-oxo-4-phenylbutyrate chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,2-oxo-4-phenylbutanoic acid ethyl ester physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,4-phenyl-2-oxobutyric acid ethyl ester Use and application,Ethyl 2-oxo-4-phenylbutyrate technical grade,usp/ep/jp grade.
Related News: “So far, the finding is still under preliminary study, and a large number of experiments is needed to test if it is effective on patients,” it said, citing the WHO in saying that no proven effective drugs to prevent or treat the Wuhan coronavirus are currently available.3-Fluoro-2-methylpyridine manufacturer Established pharmaceutical companies have abandoned the original model of the entire industry chain, stripped R & D and production links through outsourcing, and shifted their focus to product layout and global operations.2-Chloroadenosine supplier Established pharmaceutical companies have abandoned the original model of the entire industry chain, stripped R & D and production links through outsourcing, and shifted their focus to product layout and global operations.2-Methoxy-3-methylpyrazine vendor In April 2019, Glenmark had received approval from the Drugs Controller General of India (DCGI) for Remogliflozin Etabonate after successfully completing Phase-3 clinical trials.In April 2019, Glenmark had received approval from the Drugs Controller General of India (DCGI) for Remogliflozin Etabonate after successfully completing Phase-3 clinical trials.

