We serve Acetyl Hexapeptide-1 CAS:448944-47-6 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
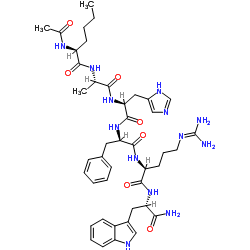
Contact us for information like Acetyl Hexapeptide-1 chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,Melitane physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,Acetyl Hexapeptide-1 Use and application,(S)-N-((6S,9R,12S,15S)-12-((1H-imidazol-5-yl)methyl)-1-amino-6-(((S)-1-amino-3-(1H-indol-3-yl)-1-oxopropan-2-yl)carbamoyl)-9-benzyl-1-imino-8,11,14-trioxo-2,7,10,13-tetraazahexadecan-15-yl)-2-acetamidohexanamide technical grade,usp/ep/jp grade.
Related News: The drug review center will establish a registration platform and database for APIs, pharmaceutical excipients and pharmaceutical packaging materials, which means that the domestic drug substance DMF system is expected to be gradually implemented.3-(Trifluoromethoxy)benzoic acid manufacturer The drug review center will establish a registration platform and database for APIs, pharmaceutical excipients and pharmaceutical packaging materials, which means that the domestic drug substance DMF system is expected to be gradually implemented.(4S)-2-oxo-3-phenylmethoxycarbonylimidazolidine-4-carboxylic acid supplier Biogen last month revived its plans to seek U.S. approval for its aducanumab treatment after announcing in March that it would terminate two large clinical trials for the drug. But some analysts believed FDA approval is highly unlikely.(2S)-2-[[(2S)-2-aminopropanoyl]amino]-3-phenylpropanoic acid vendor Biogen last month revived its plans to seek U.S. approval for its aducanumab treatment after announcing in March that it would terminate two large clinical trials for the drug. But some analysts believed FDA approval is highly unlikely.From the perspective of the overall life cycle of generic drugs from R & D to sales, with the expiration of patents, the business model for the production and marketing of generic pharmaceutical raw materials has changed, demand has increased, business potential has been realized quickly, and gross profit margins have shown a downward trend.

