We serve allyl alcohol CAS:107-18-6 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
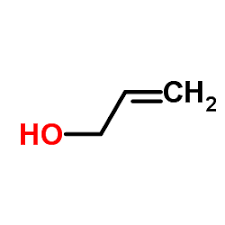
Contact us for information like allyl alcohol chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,Allylalcohol physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,2-propen-1-ol Use and application,2-propen-1-ol technical grade,usp/ep/jp grade.
Related News: After testing, our quality assurance staff confirms that everything has been performed correctly in accordance with GMP from manufacturing of API to quality testing.1-pyridin-3-ylethanone manufacturer After testing, our quality assurance staff confirms that everything has been performed correctly in accordance with GMP from manufacturing of API to quality testing.3,3-Dimethyl-1-(1H-1,2,4-triazol-1-yl)-2-butanone supplier China is fast-tracking approval for innovative drugs at home in a bid to offer more and cheaper options to patients, as many in the rapidly aging country struggle to find alternatives to costly treatments sold by multinational pharmaceutical firms for chronic diseases.2-Formylbenzenesulfonic Acid Sodium Salt vendor China is fast-tracking approval for innovative drugs at home in a bid to offer more and cheaper options to patients, as many in the rapidly aging country struggle to find alternatives to costly treatments sold by multinational pharmaceutical firms for chronic diseases.Pre-approval Access Programs (also known as expanded access, early access, compassionate use, named patient supply) are regulatory-compliant processes permitting experimental agents in development to be made available upon the request of a physician or a patient for appropriate patients for whom no alternative treatment option exists in their country.

