We serve Ammonium, hexamethylenebis[triethyl-, dibromide CAS:7072-43-7 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
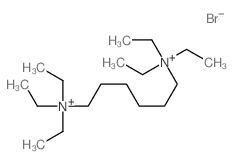
Contact us for information like Ammonium, hexamethylenebis[triethyl-, dibromide chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,1-Acetylhexahydro-1H-azepine physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,1-Acetyl-hexahydro-azepin Use and application,1-Acetyl-hexahydro-azepin technical grade,usp/ep/jp grade.
Related News: The injection has previously proven to be as effective as standard daily pills with three active ingredients when administered monthly and also once every two months.3-fluoroaniline manufacturer In addition to APIs, a variety of pharmaceutical excipients are contained in the medicine.tert-Butyl (4R-cis)-6-[(acetyloxy)methyl]-2,2-dimethyl-1,3-dioxane-4-acetate supplier In addition to APIs, a variety of pharmaceutical excipients are contained in the medicine.2-Chloro-5-nitroanisole vendor In addition to APIs, a variety of pharmaceutical excipients are contained in the medicine.Professionally produce and submit DMF files, and supplement data at any time during the patent challenge of formulation companies.

