We serve Calcium Formate CAS:544-17-2 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
Other name:
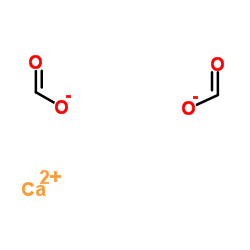
CAS No.: 544-17-2
Ca(HCOO)2
Molecular Weight: 130.12
Characters: White crystal or crystalline powder, soluble in water, insoluble in alcohols
Specifications:
| Calcium Formate | Coating grade | Feed grade | Building material grade |
| Item | Spec. | Spec. | Spec. |
| Calcium FormateAssay(Ca(HCOO)2)% | ≥99.0 | ≥98.0 | ≥97.0 |
| Total calcium(Ca)% | ≥30.4 | ≥30.1 | ≥29.8 |
| Water insoluble% | ≤0.15 | ≤0.2 | ≤0.4 |
| pH-value(50g/L) | 7.0-7.5 | 7.0-7.5 | 6.5-7.5 |
| Drying loss(as Pb) % | ≤0.002 | ≤0.002 | ≤0.004 |
| Chlorides(Cl) % | ≤0.05 | — | — |
| (As)% | ≤0.0005 | ≤0.0005 | ≤0.001 |
Product Usage: Used for feed additives and buildings.
Packaging: In 25Kg/woven bag lined with double-layer plastic bag
Storage Precautions: Stored in cool, draughty and dry place at room temperature.
Contact us for information like N-butyl pyridinium bromide chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,Formic acid,berylliumsalt (8CI,9CI) physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,calcium diformate Use and application,Pyruvonitril technical grade,usp/ep/jp grade.
Related News: Zhejiang Tianyu Co., Ltd .: It is mainly engaged in the research and development and production of sartan-type APIs and intermediates. It has serialized sartan-type APIs and intermediate products, and has accumulated rich chemical synthesis technology. It has strong technical advantages and Scale advantage.1-diphenylphosphoryloxy-2,3,4,5,6-pentafluorobenzene manufacturer Inceptua is a pharmaceutical company and service partner spanning throughout the product lifecycle – from comparator sourcing for clinical trials, through early access programs to licensing and commercialization for products.tetrapropan-2-yl silicate supplier Pre-approval Access Programs (also known as expanded access, early access, compassionate use, named patient supply) are regulatory-compliant processes permitting experimental agents in development to be made available upon the request of a physician or a patient for appropriate patients for whom no alternative treatment option exists in their country.2-nitro-p-toluidine vendor Pre-approval Access Programs (also known as expanded access, early access, compassionate use, named patient supply) are regulatory-compliant processes permitting experimental agents in development to be made available upon the request of a physician or a patient for appropriate patients for whom no alternative treatment option exists in their country.Under the high pressure of environmental protection, if Chinese API companies want to achieve sustainable operation, they must recognize the current situation, increase environmental investment, and carry out industrial upgrades, focusing on new product development, process improvement, CMO business and new technology research. And other businesses to carry out work to improve safety, environmental protection, quality, cost and other capabilities, so as to seize structural opportunities.

