We serve Chemical Name:dichlorovanadium CAS:10580-52-6 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
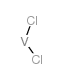
Chemical Name:dichlorovanadium
CAS.NO:10580-52-6
Synonyms:EINECS 234-176-7;MFCD00043084;vanadium dichloride
Molecular Formula:Cl2V
Molecular Weight:121.84700
HS Code:
Physical and Chemical Properties:
Melting point:910ºC
Boiling point:910°C
Density:3.09 g/mL at 25 °C(lit.)
Index of Refraction:
PSA:
Exact Mass:120.88200
LogP:1.37900
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:UN 3260 8/PG 2
Packing Group:III
Contact us for information like EINECS 234-176-7 chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,vanadium dichloride physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,MFCD00043084 Use and application,EINECS 234-176-7 technical grade,usp/ep/jp grade.
Related News: At present, there are more than 8,000 domestic API manufacturers, but they mainly produce bulk APIs with low technical content. dichlorovanadium manufacturer Therefore, the R & D capabilities and registration capabilities of API companies have become the core factors of competition. dichlorovanadium supplier Lilly has pledged to address the problems, and said none of them affected drugs in the market. The FDA, which has taken no public action, did not respond to requests for comment. dichlorovanadium vendor Capacity: the bottleneck of business growth, expanding capacity and increasing utilization become a trend. Capacity is the core factor that determines the supply capacity of API companies. Insufficient capacity will cause companies to selectively complete orders and produce varieties, limiting the expansion of API products and market development. dichlorovanadium factory Lilly has pledged to address the problems, and said none of them affected drugs in the market. The FDA, which has taken no public action, did not respond to requests for comment.

