We serve Chemical Name:4-(quinolin-8-ylsulfonylamino)benzoic acid CAS:116834-64-1 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
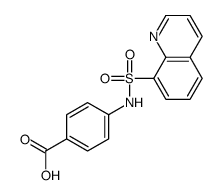
Chemical Name:4-(quinolin-8-ylsulfonylamino)benzoic acid
CAS.NO:116834-64-1
Synonyms:4-(quinolin-8-ylsulfonylamino)benzoic acid
Molecular Formula:C16H12N2O4S
Molecular Weight:328.34200
HS Code:
Physical and Chemical Properties:
Melting point:N/A
Boiling point:N/A
Density:N/A
Index of Refraction:
PSA:104.74000
Exact Mass:328.05200
LogP:3.88760
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like 4-(quinolin-8-ylsulfonylamino)benzoic acid chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,4-(quinolin-8-ylsulfonylamino)benzoic acid physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,4-(quinolin-8-ylsulfonylamino)benzoic acid Use and application,4-(quinolin-8-ylsulfonylamino)benzoic acid technical grade,usp/ep/jp grade.
Related News: Since the listing of high-barrier generic drugs involves quickly bypassing or challenging process patents of original research products within a short period of time, API companies play an important role in the process of marketing and sales of generic drugs. 4-(quinolin-8-ylsulfonylamino)benzoic acid manufacturer Patients given the vaccine also experienced a reduction in cerebrospinal fluid biomarkers of abnormal tau, results show. 4-(quinolin-8-ylsulfonylamino)benzoic acid supplier In an August overhaul to its drug administration law, Beijing said conditional approval could be granted to some still-under-research medicines of ��predictable�� clinical value for life-threatening diseases for which effective treatment is not immediately available. 4-(quinolin-8-ylsulfonylamino)benzoic acid vendor In an August overhaul to its drug administration law, Beijing said conditional approval could be granted to some still-under-research medicines of ��predictable�� clinical value for life-threatening diseases for which effective treatment is not immediately available. 4-(quinolin-8-ylsulfonylamino)benzoic acid factory The clinical trial International Study of Phase 3 IV RigosErtib, or INSPIRE, was finalized following guidance received from the U.S. Food and Drug Administration and European Medicines Agency.

