We serve Chemical Name:tert-butyl N-(4-cyanopiperidin-4-yl)carbamate CAS:1205749-01-4 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
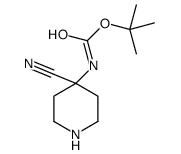
Chemical Name:tert-butyl N-(4-cyanopiperidin-4-yl)carbamate
CAS.NO:1205749-01-4
Synonyms:tert-butyl N-(4-cyanopiperidin-4-yl)carbamate
Molecular Formula:C11H19N3O2
Molecular Weight:225.28700
HS Code:2933399090
Physical and Chemical Properties:
Melting point:N/A
Boiling point:N/A
Density:N/A
Index of Refraction:
PSA:77.64000
Exact Mass:225.14800
LogP:1.69008
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like tert-butyl N-(4-cyanopiperidin-4-yl)carbamate chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,tert-butyl N-(4-cyanopiperidin-4-yl)carbamate physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,tert-butyl N-(4-cyanopiperidin-4-yl)carbamate Use and application,tert-butyl N-(4-cyanopiperidin-4-yl)carbamate technical grade,usp/ep/jp grade.
Related News: The quality of the drug substance determines the quality of the preparation, so its quality standards are very strict. Countries around the world have formulated strict national pharmacopoeia standards and quality control methods for their widely used drug substances. tert-butyl N-(4-cyanopiperidin-4-yl)carbamate manufacturer Onconova Therapeutics, Inc. and Inceptua Medicines Access (a business unit of the Inceptua Group) recently announced they have entered into a collaboration to make available intravenous rigosertib via a Pre-approval Access Program in selected countries around the world. tert-butyl N-(4-cyanopiperidin-4-yl)carbamate supplier The intravenous form of rigosertib has been studied in Phase 1, 2, and 3 clinical trials involving more than 1000 patients, and is currently being evaluated in a randomized Phase 3 international INSPIRE trial for patients with HR-MDS after failure of HMA therapy. tert-butyl N-(4-cyanopiperidin-4-yl)carbamate vendor Onconova Therapeutics, Inc. and Inceptua Medicines Access (a business unit of the Inceptua Group) recently announced they have entered into a collaboration to make available intravenous rigosertib via a Pre-approval Access Program in selected countries around the world. tert-butyl N-(4-cyanopiperidin-4-yl)carbamate factory Onconova Therapeutics, Inc. and Inceptua Medicines Access (a business unit of the Inceptua Group) recently announced they have entered into a collaboration to make available intravenous rigosertib via a Pre-approval Access Program in selected countries around the world.

