We serve Chemical Name:1,13-dioxa-4,7,10,16,19,22-hexazacyclotetracosane,hydrochloride CAS:126875-53-4 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
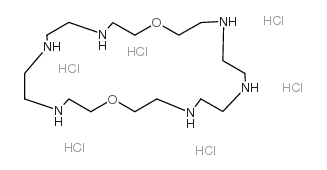
Chemical Name:1,13-dioxa-4,7,10,16,19,22-hexazacyclotetracosane,hydrochloride
CAS.NO:126875-53-4
Synonyms:1,13-Dioxa-4,7,10,16,19,22-hexaaza-cyclotetracosane hydrochloride;1,4,10,13,16,22-hexaaza-7,19-dioxacyclotetracosane hexahydrochloride;1,13-dioxa-4,7,10,16,19,22-hexazacyclotetracosane hydrochloride
Molecular Formula:C16H44Cl6N6O2
Molecular Weight:565.27800
HS Code:
Physical and Chemical Properties:
Melting point:N/A
Boiling point:671.3ºC at 760mmHg
Density:N/A
Index of Refraction:
PSA:90.64000
Exact Mass:562.16600
LogP:4.35560
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like 1,13-Dioxa-4,7,10,16,19,22-hexaaza-cyclotetracosane hydrochloride chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,1,13-dioxa-4,7,10,16,19,22-hexazacyclotetracosane hydrochloride physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,1,13-dioxa-4,7,10,16,19,22-hexazacyclotetracosane hydrochloride Use and application,1,13-Dioxa-4,7,10,16,19,22-hexaaza-cyclotetracosane hydrochloride technical grade,usp/ep/jp grade.
Related News: Compared with individuals with cancer only, those with ASCVD and with ASCVD and cancer had a significantly higher presence of three of more of these factors (23 and 30 percent, respectively, versus 13 percent). 1,13-dioxa-4,7,10,16,19,22-hexazacyclotetracosane,hydrochloride manufacturer Compared with individuals with cancer only, those with ASCVD and with ASCVD and cancer had a significantly higher presence of three of more of these factors (23 and 30 percent, respectively, versus 13 percent). 1,13-dioxa-4,7,10,16,19,22-hexazacyclotetracosane,hydrochloride supplier First of all, as an API manufacturer we think of how to make a chemical compound which becomes an API in the laboratory. 1,13-dioxa-4,7,10,16,19,22-hexazacyclotetracosane,hydrochloride vendor From the perspective of the corresponding formulation manufacturer, the drug substance needs to meet the requirements of impurities and stability. The production base must pass the international quality system certifications such as cGMP and EuGMP. At the same time, the drug substance company must have sufficient capacity. 1,13-dioxa-4,7,10,16,19,22-hexazacyclotetracosane,hydrochloride factory Compared with existing Imbruvica therapies, where patients take the BTK inhibitor daily—potentially for years—until their disease progresses, the new Venclexta combo caps treatment at about 14 months. That could offer a new option for “probably younger patients with CLL that prefer more flexible, treatment-free intervals,” said Craig Tendler, M.D., vice president of oncology clinical development and global medical affairs at J&J’s Janssen unit, during an interview.

