We serve Chemical Name:Verteporfin CAS:129497-78-5 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
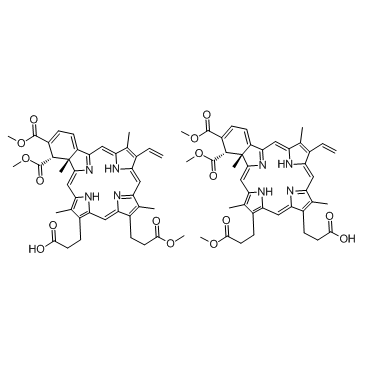
Chemical Name:Verteporfin
CAS.NO:129497-78-5
Synonyms:trans-3,4-Dicarboxy-4,4a-dihydro-4a,8,14,19-tetramethyl-18-vinyl-23H,25H-benzo(b)porphine-9,13-dipropionic acid 3,4,9-trimethyl ester;Visudyne;,3(28),4,6,8,10,12,14,16(26),17,19,21-dodécaén-9-yl]propanoïque – acide 3-[(1Z,6Z,12Z,17Z,23S,24R)-14-éthényl-22,23-bis(méthoxycarbonyl)-9-(3-méthoxy-3-oxopropyl)-4,10,15,24-tétraméthyl-25,26,27,28-té;VERTEPORFIN (200 MG);trans-18-Ethenyl-4,4a-dihydro-3,4-bis(methoxycarbonyl)-4a,8,14,19-tetramethyl-23H,25H-benzo[b]porphine-9,13-dipropionic Acid Monomethyl Ester;,4,6,8,10,12,14,16(26),17,19,21-dodecaen-9-yl]propanoic acid – 3-[(1Z,6Z,12Z,17Z,23S,24R)-14-ethenyl-22,23-bis(methoxycarbonyl)-9-(3-methoxy-3-oxopropyl)-4,10,15,24-tetramethyl-25,26,27,28-tetraazahex;,4,6,8,10,12,14,16(26),17,19,21-dodecaen-9-yl]propansäure–3-[(1Z,6Z,12Z,17Z,23S,24R)-14-ethenyl-22,23-bis(methoxycarbonyl)-9-(3-methoxy-3-oxopropyl)-4,10,15,24-tetramethyl-25,26,27,28-tetraazahexacyc;Verteporphin;3-[(1Z,6Z,12Z,17Z,23S,24R)-22,23-Bis(methoxycarbonyl)-5-(3-methoxy-3-oxopropyl)-4,10,15,24-tetramethyl-14-vinyl-25,26,27,28-tetraazahexacyclo[16.6.1.1.1.1.0]octacosa-1,3(28),4 ,6,8,10,12,14,16(26),17,19,21-dodecaen-9-yl]propanoic acid – 3-[(1Z,6Z,12Z,17Z,23S,24R)-22,23-bis(methoxycarbonyl)-9-(3-methoxy-3-oxopropyl)-4,10,15,24-tetramethyl-14-vinyl-25,26,27,28-tetraazahexacyc lo[16.6.1.1.1.1.0]o;Verteprofin
Molecular Formula:C41H42N4O8
Molecular Weight:718.79
HS Code:
Physical and Chemical Properties:
Melting point:N/A
Boiling point:N/A
Density:N/A
Index of Refraction:
PSA:337.48000
Exact Mass:
LogP:10.24600
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:NONH for all modes of transpor
Packing Group:
Contact us for information like trans-3,4-Dicarboxy-4,4a-dihydro-4a,8,14,19-tetramethyl-18-vinyl-23H,25H-benzo(b)porphine-9,13-dipropionic acid 3,4,9-trimethyl ester chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,Verteprofin physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,Verteprofin Use and application,,3(28),4,6,8,10,12,14,16(26),17,19,21-dodécaén-9-yl]propanoïque – acide 3-[(1Z,6Z,12Z,17Z,23S,24R)-14-éthényl-22,23-bis(méthoxycarbonyl)-9-(3-méthoxy-3-oxopropyl)-4,10,15,24-tétraméthyl-25,26,27,28-té technical grade,usp/ep/jp grade.
Related News: Fate Therapeutics�� iPSC product platform is supported by an intellectual property portfolio of over 250 issued patents and 150 pending patent applications. Verteporfin manufacturer Though Innova previously applied for a regulatory green light, the test has not been authorized or approved by the FDA for use in the U.S.—however, during inspections of the company’s California facilities in March and April, FDA investigators said they found the test was already being sold and distributed. Verteporfin supplier Beneficial drug raw materials refer to the active pharmaceutical ingredients used in the manufacture of original research drugs (patent drugs). They are mainly used to meet the needs of original multinational pharmaceutical companies and emerging biopharmaceutical companies for innovative drugs in clinical drug research, registration approval and commercialization sales , Which also contains advanced intermediates used in the manufacture of the drug substance that need to be regulated by regulatory authorities. Verteporfin vendor Though Innova previously applied for a regulatory green light, the test has not been authorized or approved by the FDA for use in the U.S.—however, during inspections of the company’s California facilities in March and April, FDA investigators said they found the test was already being sold and distributed. Verteporfin factory Though Innova previously applied for a regulatory green light, the test has not been authorized or approved by the FDA for use in the U.S.—however, during inspections of the company’s California facilities in March and April, FDA investigators said they found the test was already being sold and distributed.

