We serve Chemical Name:N-Vinylformamide CAS:13162-05-5 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
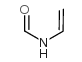
Chemical Name:N-Vinylformamide
CAS.NO:13162-05-5
Synonyms:VFA;N-VinylforMaMide;N-ethenyl-Formamide;N-vinyl-formamide;n-ethenyl-formamid;N-formyl-vinylamine;vinylformamide;EINECS 236-102-9;ethenyl formamide;SR-497;MFCD00081206
Molecular Formula:C3H5NO
Molecular Weight:71.07790
HS Code:2924199090
Physical and Chemical Properties:
Melting point:-16ºC(lit.)
Boiling point:210ºC(lit.)
Density:1.014 g/mL at 25ºC(lit.)
Index of Refraction:n20/D 1.494(lit.)
PSA:29.10000
Exact Mass:71.03710
LogP:0.90270
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:NONH for all modes of transpor
Packing Group:
Contact us for information like VFA chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,MFCD00081206 physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,vinylformamide Use and application,MFCD00081206 technical grade,usp/ep/jp grade.
Related News: Specifically, the EC has cleared Verquvo (vericiguat) to treat patients who are stabilised after a recent decompensation event requiring intravenous therapy. 5-ethyl-2-methoxybicyclo[2.2.1]heptane manufacturers Except current data is insufficient to determine its benefit in patients who are negative for anti-AChR antibodies. Benzyloxycarbonyl-ω-amino-undecansaeure-thiophenylester suppliers These have since been joined by many other types of enzyme, including transaminases, oxidases, nitrilases, aldolases, and ammonia lyases, all of which perform distinct functions. ((9r,10r)-9,10-dihydro-9,10-ethanoanthracene-11,12-diyl)bis(methylene) diacetate vendor & factory Specifically, the EC has cleared Verquvo (vericiguat) to treat patients who are stabilised after a recent decompensation event requiring intravenous therapy.,The CDC’s delay likely came as a disappointment to representatives for the immunocompromised community, who pleaded with the CDC’s expert group to give the go-ahead for booster use in these people, who do not mount a strong enough defense against the virus.

