We serve Chemical Name:Barium sulphate CAS:13462-86-7 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
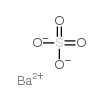
Chemical Name:Barium sulphate
CAS.NO:13462-86-7
Synonyms:Barium sulfate,natural;Barium sulphate;Einecs 236-664-5
Molecular Formula:BaO4S
Molecular Weight:233.39000
HS Code:2833270000
Physical and Chemical Properties:
Melting point:1580ºC
Boiling point:330ºC at 760 mmHg
Density:4.25-4.5
Index of Refraction:
PSA:88.64000
Exact Mass:233.85700
LogP:
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like Barium sulfate,natural chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,Einecs 236-664-5 physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,Einecs 236-664-5 Use and application,Einecs 236-664-5 technical grade,usp/ep/jp grade.
Related News: While they are early data, “it’s also very reassuring that those patients who have progressed can be rather easily reinduced into remission,” Tendler said. J&J and AbbVie are now taking the same approach for the GLOW study, he added. Barium sulphate manufacturer The move follows a Class I recall, the FDA’s most serious, launched by Innova in late April amid “significant concerns” about the test’s accuracy—and alongside an official warning letter delivered to the company this week. Barium sulphate supplier The move could force international drugmakers to further cut prices and enable copycat medicines to replace imported off-patent brands at faster pace. Barium sulphate vendor Singapore, Australia, New Zealand, and the United States have banned all foreign nationals traveling from China from entering the country. Barium sulphate factory The move follows a Class I recall, the FDA’s most serious, launched by Innova in late April amid “significant concerns” about the test’s accuracy—and alongside an official warning letter delivered to the company this week.

