We serve Chemical Name:Trichlororhodium hexaammoniate CAS:13820-96-7 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
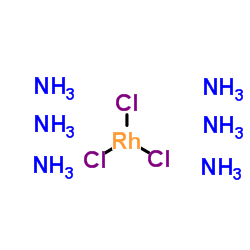
Chemical Name:Trichlororhodium hexaammoniate
CAS.NO:13820-96-7
Synonyms:Hexaamminerhodium trichloride;Hexaamminerhodium(III) chloride;Trichlororhodium hexaammoniate;EINECS 237-506-8
Molecular Formula:Cl3H18N6Rh
Molecular Weight:311.448
HS Code:
Physical and Chemical Properties:
Melting point:N/A
Boiling point:N/A
Density:N/A
Index of Refraction:
PSA:19.44000
Exact Mass:309.971344
LogP:4.01190
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like Hexaamminerhodium trichloride chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,EINECS 237-506-8 physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,Hexaamminerhodium trichloride Use and application,Hexaamminerhodium(III) chloride technical grade,usp/ep/jp grade.
Related News: This dosage form may also support combination therapy modalities.? To date, over 400 patients have been dosed with the oral formulation of rigosertib in clinical trials.? Combination therapy of oral rigosertib with azacitidine, the standard of care in HR-MDS, has also been studied. Currently, oral rigosertib is being developed as a combination therapy together with azacitidine for patients with higher-risk MDS who require HMA therapy.? Trichlororhodium hexaammoniate manufacturer In a stringent xenograft model of disseminated lymphoblastic leukemia, FT819 demonstrated enhanced tumor clearance and control of leukemia as compared to primary CAR19 T cells. Trichlororhodium hexaammoniate supplier In a stringent xenograft model of disseminated lymphoblastic leukemia, FT819 demonstrated enhanced tumor clearance and control of leukemia as compared to primary CAR19 T cells. Trichlororhodium hexaammoniate vendor This dosage form may also support combination therapy modalities.? To date, over 400 patients have been dosed with the oral formulation of rigosertib in clinical trials.? Combination therapy of oral rigosertib with azacitidine, the standard of care in HR-MDS, has also been studied. Currently, oral rigosertib is being developed as a combination therapy together with azacitidine for patients with higher-risk MDS who require HMA therapy.? Trichlororhodium hexaammoniate factory This dosage form may also support combination therapy modalities.? To date, over 400 patients have been dosed with the oral formulation of rigosertib in clinical trials.? Combination therapy of oral rigosertib with azacitidine, the standard of care in HR-MDS, has also been studied. Currently, oral rigosertib is being developed as a combination therapy together with azacitidine for patients with higher-risk MDS who require HMA therapy.?

