We serve Chemical Name:manganese molybdate CAS:14013-15-1 to global customers since 2007, Pls send inquiry to info@nbinno.com or visit www.nbinno.com our official website should you have any interests. This site is for information only.
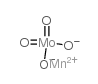
Chemical Name:manganese molybdate
CAS.NO:14013-15-1
Synonyms:Manganese molybdenum oxide;MANGANESE (II) MOLYBDATE;manganese molybdenum tetraoxide;manganesemolybdenumoxide(mnmoo4);Molybdic acid manganese(II) salt;Manganesemolybdateoffwhitepowder;Manganese (II) molybdenum oxide;MFCD00054005;EINECS 237-823-1
Molecular Formula:MnMoO4
Molecular Weight:214.89600
HS Code:
Physical and Chemical Properties:
Melting point:>350ºC(lit.)
Boiling point:N/A
Density:N/A
Index of Refraction:
PSA:80.26000
Exact Mass:216.82300
LogP:
Material Safety Information (Applicable for Hazard Chemicals)
RIDADR:
Packing Group:
Contact us for information like Manganese molybdenum oxide chemical properties,Structure,melting point,boiling point,density,molecular formula,molecular weight,EINECS 237-823-1 physical properties,toxicity information,customs codes,safety, risk, hazard and MSDS, CAS,cas number,manganese molybdenum tetraoxide Use and application,Manganese molybdenum oxide technical grade,usp/ep/jp grade.
Related News: During analysis of the clinical trial data, the researchers realized that about a third of the participants had low levels of abnormal tau protein, which makes them not very suitable for evaluating the effects of a treatment halting the progression of tau pathology. manganese molybdate manufacturer In addition, due to the complex process of changing the API supplier and the risk of uncertainty, the viscosity of pharmaceutical and specialty API companies is usually better. manganese molybdate supplier South Korea said on Sunday that it would deny entry to any foreigners who have traveled to Wuhan and surrounding Hubei Province in China in the past 14 days. manganese molybdate vendor From the perspective of the corresponding formulation manufacturer, the drug substance needs to meet the requirements of impurities and stability. The production base must pass the international quality system certifications such as cGMP and EuGMP. At the same time, the drug substance company must have sufficient capacity. manganese molybdate factory In addition, due to the complex process of changing the API supplier and the risk of uncertainty, the viscosity of pharmaceutical and specialty API companies is usually better.

